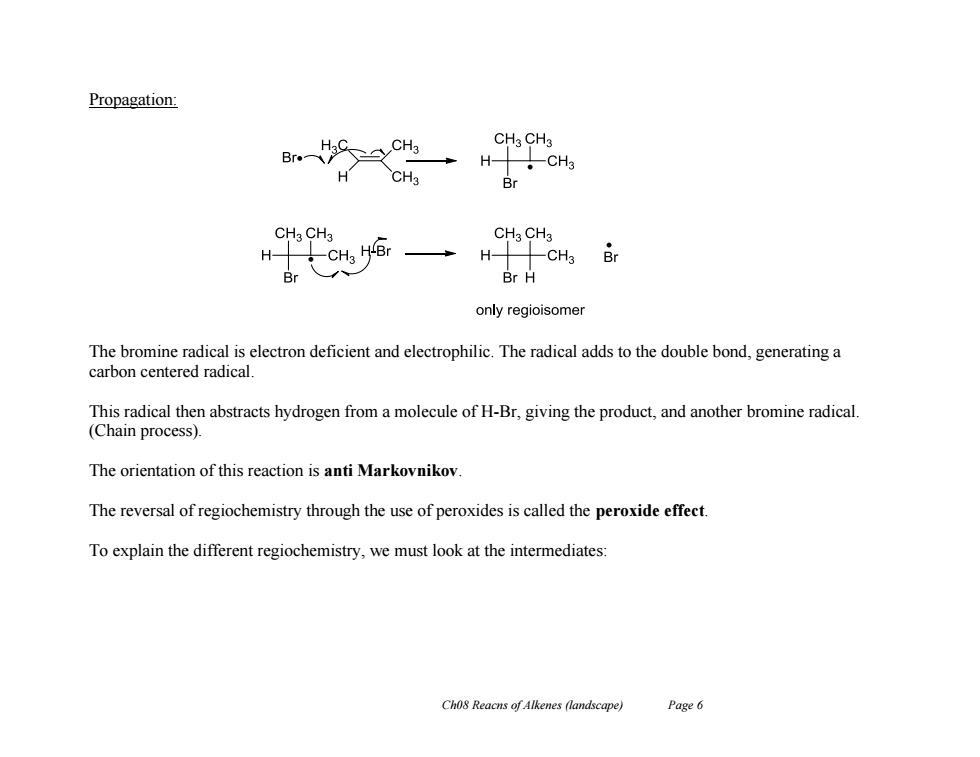
Propagation: CHa CH3 Br H CH3 H-11CH Br CHa CH3 CHaCH3 H十CH 5 Br Br H only regioisomer The bromine radical is electron deficient and electrophilic.The radical adds to the double bond,generating a carbon centered radical. This radical then abstracts hydrogen from a molecule of H-Br,giving the product,and another bromine radical. (Chain process). The orientation of this reaction is anti Markovnikov The reversal of regiochemistry through the use of peroxides is called the peroxide effect. To explain the different regiochemistry,we must look at the intermediates: Ch08 Reacns of Alkenes (landscape) Page 6
Ch08 Reacns of Alkenes (landscape) Page 6 Propagation: The bromine radical is electron deficient and electrophilic. The radical adds to the double bond, generating a carbon centered radical. This radical then abstracts hydrogen from a molecule of H-Br, giving the product, and another bromine radical. (Chain process). The orientation of this reaction is anti Markovnikov. The reversal of regiochemistry through the use of peroxides is called the peroxide effect. To explain the different regiochemistry, we must look at the intermediates:
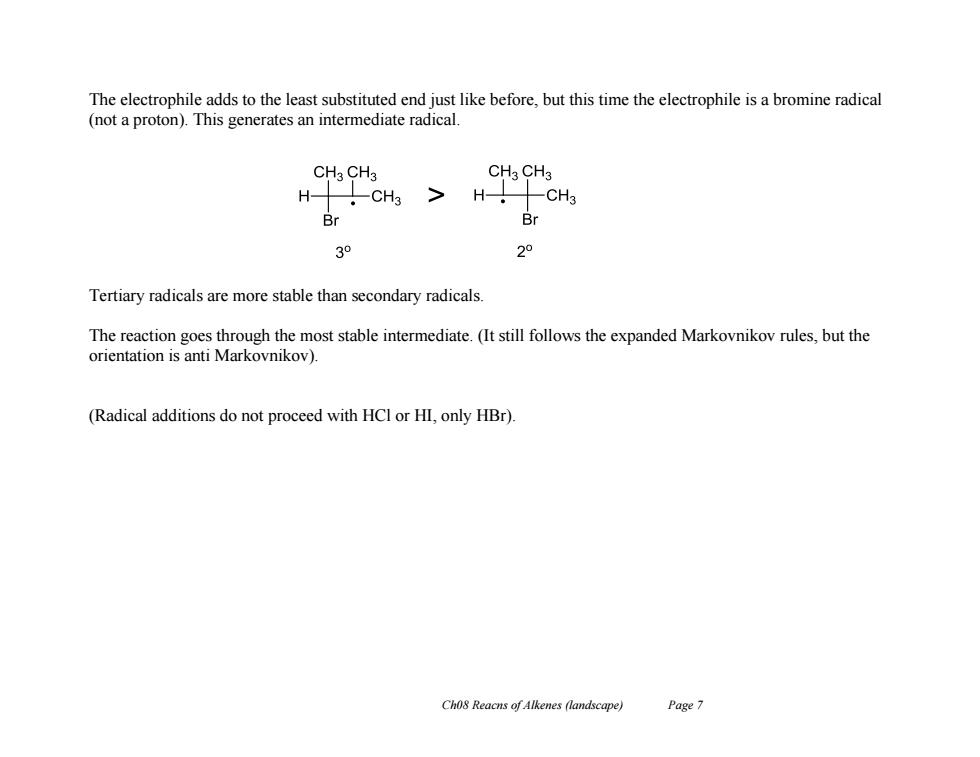
The electrophile adds to the least substituted end just like before,but this time the electrophile is a bromine radical (not a proton).This generates an intermediate radical. CH3 CH3 CH3 CH3 H H- CH3 Br 03o 2° Tertiary radicals are more stable than secondary radicals. The reaction goes through the most stable intermediate.(It still follows the expanded Markovnikov rules,but the orientation is anti Markovnikov). (Radical additions do not proceed with HCl or HI,only HBr). Ch08 Reacns of Alkenes (landscape) Page 7
Ch08 Reacns of Alkenes (landscape) Page 7 The electrophile adds to the least substituted end just like before, but this time the electrophile is a bromine radical (not a proton). This generates an intermediate radical. Tertiary radicals are more stable than secondary radicals. The reaction goes through the most stable intermediate. (It still follows the expanded Markovnikov rules, but the orientation is anti Markovnikov). (Radical additions do not proceed with HCl or HI, only HBr)

Addition of Water Alkenes can be converted to alcohols. It is the reverse reaction of the dehydration of alcohols to give alkenes +H20= H OH The principle of microscopic reversibility states that a forward reaction and a reverse reaction taking place under the same conditions must follow the same reaction pathway in microscopic detail (Logically,it seems sensible that the lowest energy T.S.'s and intermediates for the forward reaction would be the same for the reverse reaction but in the opposite order). So it is no surprise that the mechanism for hydration of alkenes is identical to that of dehydration of alcohols,but in the reverse order of steps. Ch08 Reacns of Alkenes (landscape) Page 8
Ch08 Reacns of Alkenes (landscape) Page 8 Addition of Water Alkenes can be converted to alcohols. It is the reverse reaction of the dehydration of alcohols to give alkenes. The principle of microscopic reversibility states that a forward reaction and a reverse reaction taking place under the same conditions must follow the same reaction pathway in microscopic detail. (Logically, it seems sensible that the lowest energy T.S.’s and intermediates for the forward reaction would be the same for the reverse reaction but in the opposite order). So it is no surprise that the mechanism for hydration of alkenes is identical to that of dehydration of alcohols, but in the reverse order of steps. H OH + H2O
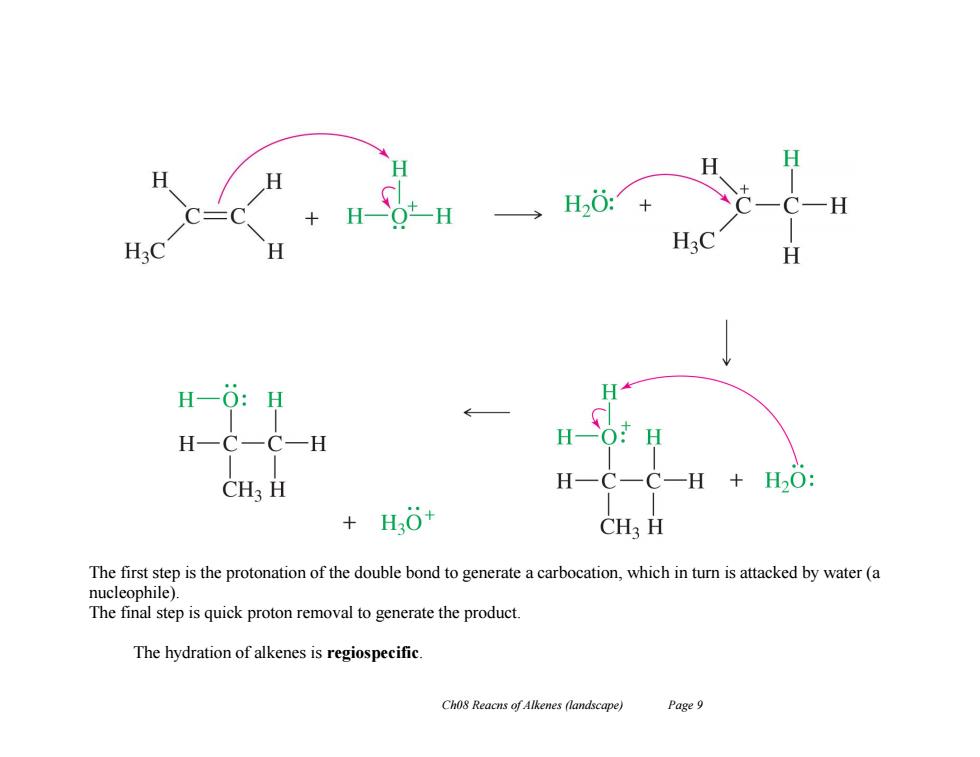
H一Ot-H H20:+ H20 H20 H H-O:H H H一C一C一H H一O:H CH3 H H-C-C一H+H2O: +H0+ CH3 H The first step is the protonation of the double bond to generate a carbocation,which in turn is attacked by water(a nucleophile). The final step is quick proton removal to generate the product. The hydration of alkenes is regiospecific. Ch08 Reacns of Alkenes (landscape) Page 9
Ch08 Reacns of Alkenes (landscape) Page 9 The first step is the protonation of the double bond to generate a carbocation, which in turn is attacked by water (a nucleophile). The final step is quick proton removal to generate the product. The hydration of alkenes is regiospecific

The orientation is Markovnikov since the proton has added to the least highly substituted end,and the hydroxyl to the most highly substituted end. The regiochemistry is explained by the intermediate carbocation: HH HH H + -CH3 H CH3 H 2° 10 The secondary carbocation is more stable than the primary carbocation The reaction of dilute acid to hydrate alkenes is not a fantastic practical route due to insolubility problems,and typically two other indirect approaches are used. (1)Addition of sulfuric acid followed by hydrolysis (2)Oxymercuration-demercuration Addition of Sulfuric Acid followed by Hydrolysis The alkene reacts with conc.sulfuric acid to give an alkyl hydrogen sulfate,which then in turn is hydrolyzed to give the alcohol. +H2SO4 H20 H OSO3H H OH alkene alkyl hydrogen alcohol sulfate Cho8 Reacns of Alkenes (landscape) Page 10
Ch08 Reacns of Alkenes (landscape) Page 10 The orientation is Markovnikov since the proton has added to the least highly substituted end, and the hydroxyl to the most highly substituted end. The regiochemistry is explained by the intermediate carbocation: The secondary carbocation is more stable than the primary carbocation. The reaction of dilute acid to hydrate alkenes is not a fantastic practical route due to insolubility problems, and typically two other indirect approaches are used. (1) Addition of sulfuric acid followed by hydrolysis (2) Oxymercuration-demercuration Addition of Sulfuric Acid followed by Hydrolysis The alkene reacts with conc. sulfuric acid to give an alkyl hydrogen sulfate, which then in turn is hydrolyzed to give the alcohol. H OSO3H + H2SO4 H OH H2O alkene alkyl hydrogen sulfate alcohol