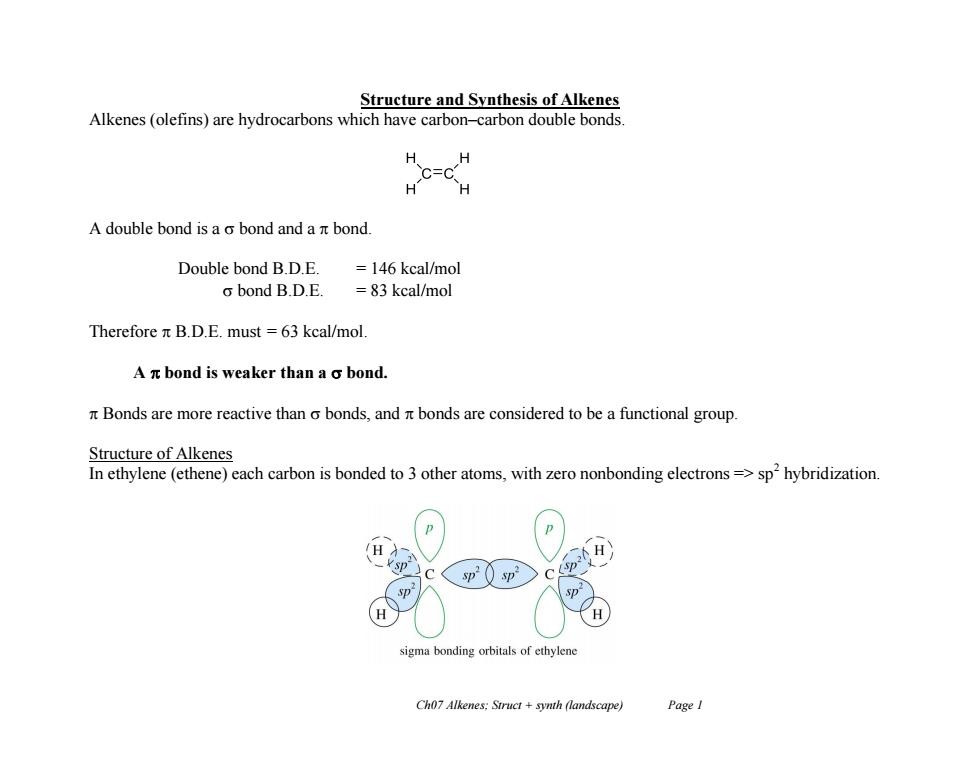
Structure and Synthesis of Alkenes Alkenes(olefins)are hydrocarbons which have carbon-carbon double bonds H H =C H- H A double bond is a o bond and a nt bond. Double bond B.D.E. =146 kcal/mol o bond B.D.E. =83 kcal/mol Therefore n B.D.E.must =63 kcal/mol. A n bond is weaker than a o bond. Bonds are more reactive than o bonds,and r bonds are considered to be a functional group. Structure of Alkenes In ethylene(ethene)each carbon is bonded to 3 other atoms,with zero nonbonding electrons=>sp'hybridization. sigma bonding orbitals of ethylene Ch07 Alkenes:Struct +synth (landscape) Page I
Ch07 Alkenes; Struct + synth (landscape) Page 1 Structure and Synthesis of Alkenes Alkenes (olefins) are hydrocarbons which have carbon–carbon double bonds. A double bond is a bond and a bond. Double bond B.D.E. = 146 kcal/mol bond B.D.E. = 83 kcal/mol Therefore B.D.E. must = 63 kcal/mol. A bond is weaker than a bond. Bonds are more reactive than bonds, and bonds are considered to be a functional group. Structure of Alkenes In ethylene (ethene) each carbon is bonded to 3 other atoms, with zero nonbonding electrons => sp2 hybridization
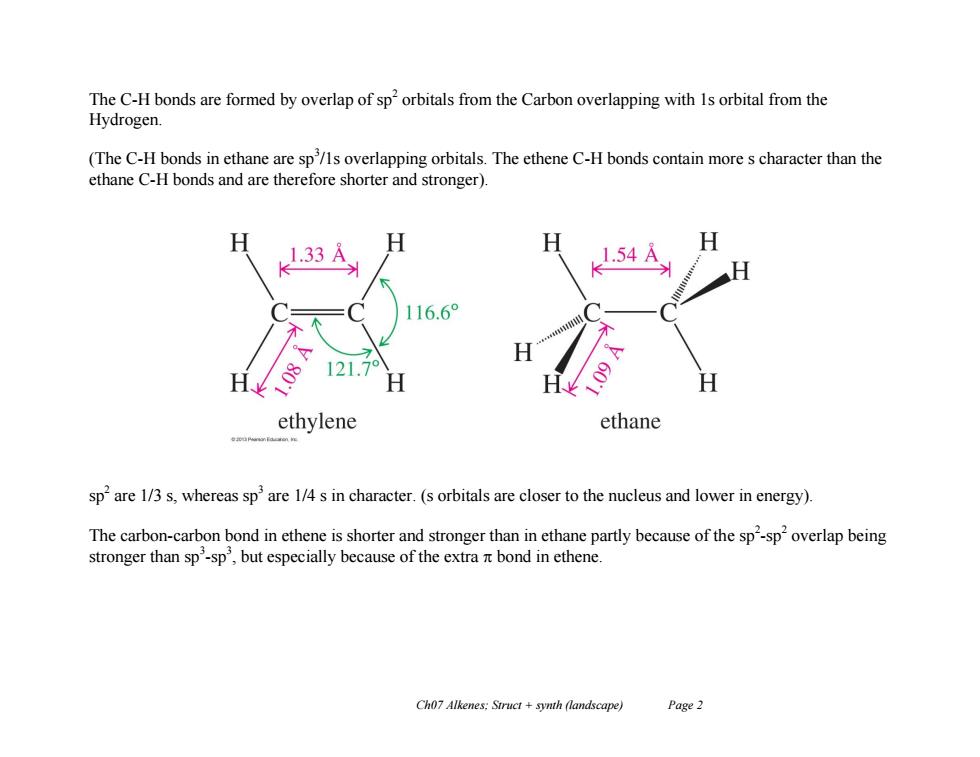
The C-H bonds are formed by overlap of sp'orbitals from the Carbon overlapping with 1s orbital from the Hydrogen. (The C-H bonds in ethane are sp/ls overlapping orbitals.The ethene C-H bonds contain more s character than the ethane C-H bonds and are therefore shorter and stronger). 1.33A H H 1.54A 116.6° 121.7 H ethylene ethane sp'are 1/3 s,whereas sp'are 1/4 s in character.(s orbitals are closer to the nucleus and lower in energy). The carbon-carbon bond in ethene is shorter and stronger than in ethane partly because of the sp'-sp overlap being stronger than sp'-sp',but especially because of the extra it bond in ethene. Ch07 Alkenes:Struct synth (landscape) Page 2
Ch07 Alkenes; Struct + synth (landscape) Page 2 The C-H bonds are formed by overlap of sp2 orbitals from the Carbon overlapping with 1s orbital from the Hydrogen. (The C-H bonds in ethane are sp3 /1s overlapping orbitals. The ethene C-H bonds contain more s character than the ethane C-H bonds and are therefore shorter and stronger). sp 2 are 1/3 s, whereas sp3 are 1/4 s in character. (s orbitals are closer to the nucleus and lower in energy). The carbon-carbon bond in ethene is shorter and stronger than in ethane partly because of the sp 2 -sp 2 overlap being stronger than sp3 -sp 3 , but especially because of the extra bond in ethene
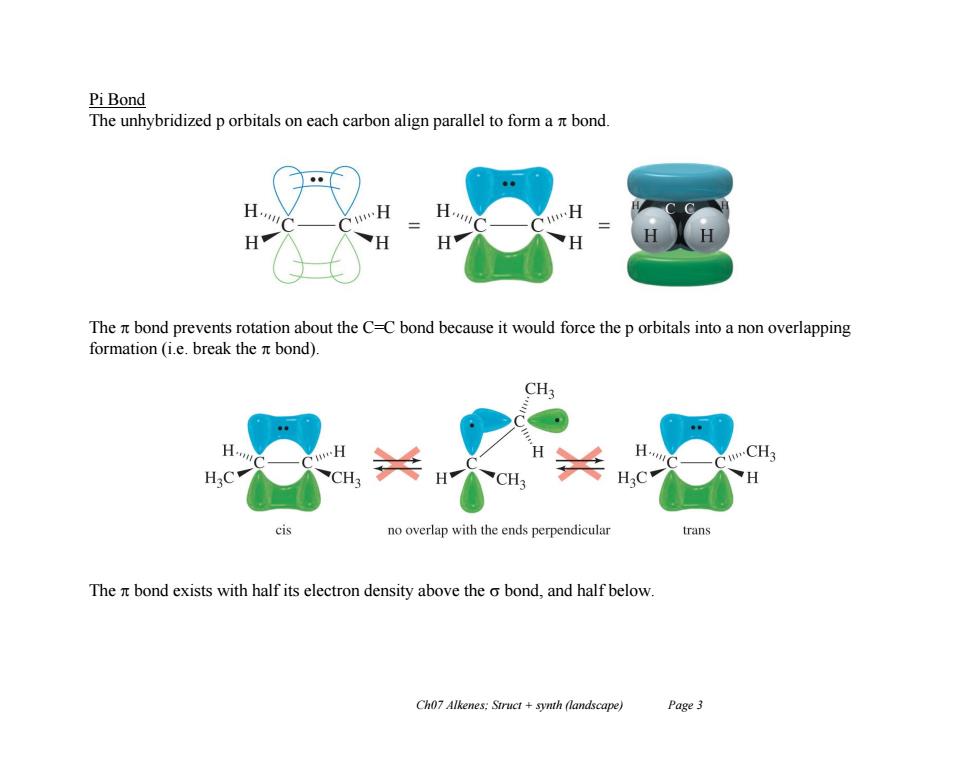
Pi Bond The unhybridized p orbitals on each carbon align parallel to form a nt bond. The n bond prevents rotation about the C-C bond because it would force the p orbitals into a non overlapping formation (i.e.break the nt bond). CH H CH3 CH CH no overlap with the ends perpendicular ran The nt bond exists with half its electron density above the o bond,and half below. Ch07 Alkenes:Struct +synth (landscape) Page3
Ch07 Alkenes; Struct + synth (landscape) Page 3 Pi Bond The unhybridized p orbitals on each carbon align parallel to form a bond. The bond prevents rotation about the C=C bond because it would force the p orbitals into a non overlapping formation (i.e. break the bond). The bond exists with half its electron density above the bond, and half below
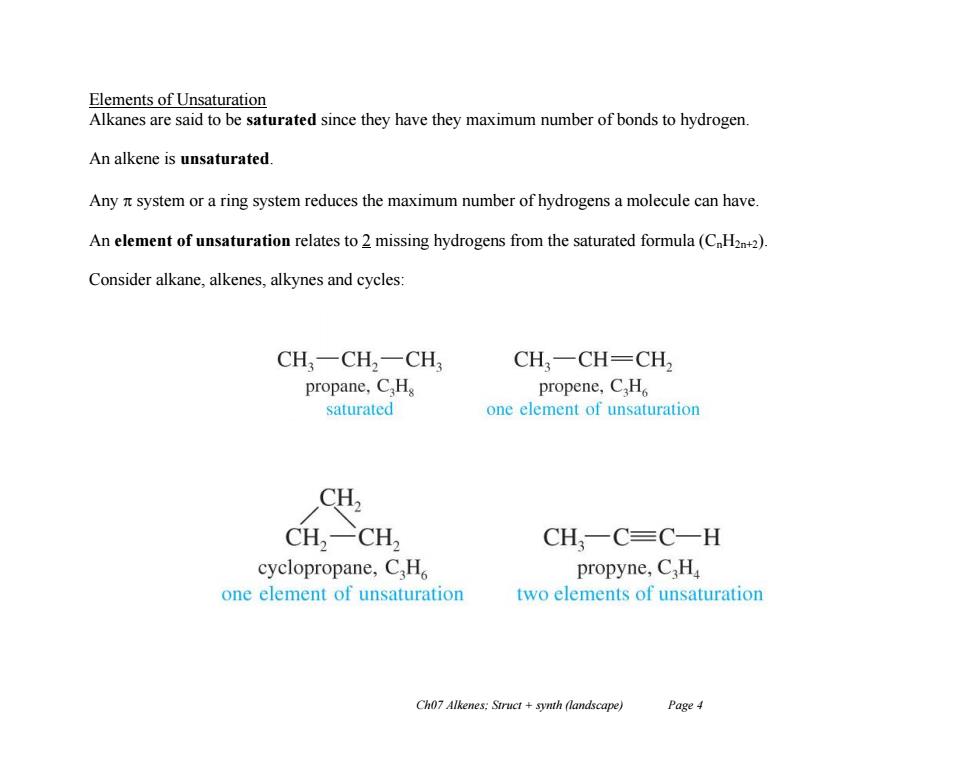
Elements of Unsaturation Alkanes are said to be saturated since they have they maximum number of bonds to hydrogen. An alkene is unsaturated. Any t system or a ring system reduces the maximum number of hydrogens a molecule can have. An element of unsaturation relates to 2 missing hydrogens from the saturated formula(C.H2+2). Consider alkane,alkenes,alkynes and cycles: CH,一CH2一CH CH,一CH=CH propane,C.H propene,C.H saturated one element of unsaturation CH2 CH2-CH2 CH,一C=C一H cyclopropane,C.H propyne,CH one element of unsaturation two elements of unsaturation Ch07 Alkenes:Struct synth (landscape) Page4
Ch07 Alkenes; Struct + synth (landscape) Page 4 Elements of Unsaturation Alkanes are said to be saturated since they have they maximum number of bonds to hydrogen. An alkene is unsaturated. Any system or a ring system reduces the maximum number of hydrogens a molecule can have. An element of unsaturation relates to 2 missing hydrogens from the saturated formula (CnH2n+2). Consider alkane, alkenes, alkynes and cycles:

(Heteroatom complications Heteroatoms are considered anything other than C or H. Halogens These simply substitute for hydrogens in the molecular formula. Therefore just like C2H6 is saturated,so is C2HF2. Oxygen CH3-CH3 is saturated(C2H) CH-O-CH is also saturated(C2HO) An oxygen can be added without requiring any additional hydrogens,so ignore the number of oxygens when calculating elements of Unsaturation. Nitrogen Nitrogen is trivalent,and when it replaces a C in a chain it requires only one hydrogen (-NH-vs.-CH2-),so nitrogens count as half a carbon Thus C.HoN is equivalent to CasHo.(i.e.one element of Unsaturation).) Ch07 Alkenes:Struct synth (landscape) Page 5
Ch07 Alkenes; Struct + synth (landscape) Page 5 (Heteroatom complications Heteroatoms are considered anything other than C or H. Halogens These simply substitute for hydrogens in the molecular formula. Therefore just like C2H6 is saturated, so is C2H4F2. Oxygen CH3-CH3 is saturated (C2H6) CH3-O-CH3 is also saturated (C2H6O) An oxygen can be added without requiring any additional hydrogens, so ignore the number of oxygens when calculating elements of Unsaturation. Nitrogen Nitrogen is trivalent, and when it replaces a C in a chain it requires only one hydrogen (-NH- vs. -CH2- ), so nitrogens count as half a carbon. Thus C4H9N is equivalent to C4.5H9. (i.e. one element of Unsaturation).)