
Organic Chemistry I Dr Alex Roche Organic chemistry is the chemistry of Carbon and its compounds. Organic molecules constitute the essence of life(fats,sugars,proteins,DNA),and also permeate our everyday lives (cotton,polyester,toothpaste,plastics,etc). Chemistry's top two commercial fields are organic dominated:Pharmaceuticals and Polymers. Organic chemistry is also easy-IF:don't fall behind do the problems understand,not memorize Notes are available at: http://crab.rutgers.edu/-alroche/ Office:SCI 311 Labs:SCI309/319/328 Tel:(856)225-6166 alroche@crab.rutgers.edu Chol Introduction (landscape) Page I
Ch01 Introduction (landscape) Page 1 Organic Chemistry I Dr Alex Roche Organic chemistry is the chemistry of Carbon and its compounds. Organic molecules constitute the essence of life (fats, sugars, proteins, DNA), and also permeate our everyday lives (cotton, polyester, toothpaste, plastics, etc). Chemistry’s top two commercial fields are organic dominated: Pharmaceuticals and Polymers. Organic chemistry is also easy - IF: don’t fall behind do the problems understand, not memorize Notes are available at: http://crab.rutgers.edu/~alroche/ Office: SCI 311 Labs: SCI 309/319/328 Tel: (856) 225-6166 alroche@crab.rutgers.edu

Structure of the Atom Atoms consist of:Protons (+ve) Neutrons(neutral) Electrons (-ve) Protons and neutrons are in the nucleus and have similar masses(1800x that of an electron). Atoms with the same number of protons but different neutrons are called ISOTOPES. E.g.C(major isotope) 1C(~1%,used in carbon NMR) C(radioactive,used in Carbon dating) Almost all the mass of an atom is in the nucleus,but it is the electrons that are involved in the chemical bonding and reactions of an atom. Electronic Structure of the Atom Electrons display wave-particle duality. Electrons are located in orbitals around a nucleus,but the Heisenberg Uncertainty Principle tells us that we cannot pinpoint exactly where the electron is. So we use the term ELECTRON DENSITY,which is the probability of finding the electron in a particular part of the orbital. ORBITAL:is an allowed energy state for an electron,with an associated probability function that defines the distribution of electron density in space. Chol Introduction (landscape) Page 2
Ch01 Introduction (landscape) Page 2 Structure of the Atom Atoms consist of: Protons (+ve) Neutrons (neutral) Electrons (-ve) Protons and neutrons are in the nucleus and have similar masses (1800x that of an electron). Atoms with the same number of protons but different neutrons are called ISOTOPES. E.g. 12C (major isotope) 13C (~1%, used in carbon NMR) 14C (radioactive, used in Carbon dating) Almost all the mass of an atom is in the nucleus, but it is the electrons that are involved in the chemical bonding and reactions of an atom. Electronic Structure of the Atom Electrons display wave-particle duality. Electrons are located in orbitals around a nucleus, but the Heisenberg Uncertainty Principle tells us that we cannot pinpoint exactly where the electron is. So we use the term ELECTRON DENSITY, which is the probability of finding the electron in a particular part of the orbital. ORBITAL: is an allowed energy state for an electron, with an associated probability function that defines the distribution of electron density in space
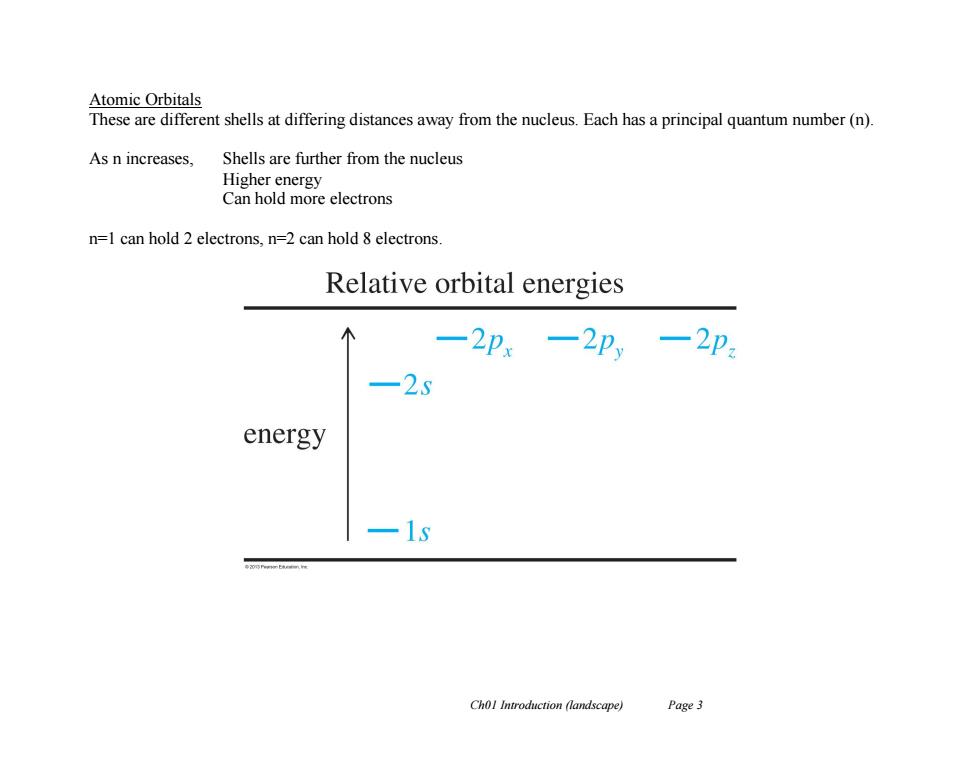
Atomic Orbitals These are different shells at differing distances away from the nucleus.Each has a principal quantum number(n). As n increases, Shells are further from the nucleus Higher energy Can hold more electrons n=1 can hold 2 electrons,n=2 can hold 8 electrons. Relative orbital energies 一2p.一2p,一2p 25 energy 1g Chol Introduction (landscape) Page3
Ch01 Introduction (landscape) Page 3 Atomic Orbitals These are different shells at differing distances away from the nucleus. Each has a principal quantum number (n). As n increases, Shells are further from the nucleus Higher energy Can hold more electrons n=1 can hold 2 electrons, n=2 can hold 8 electrons
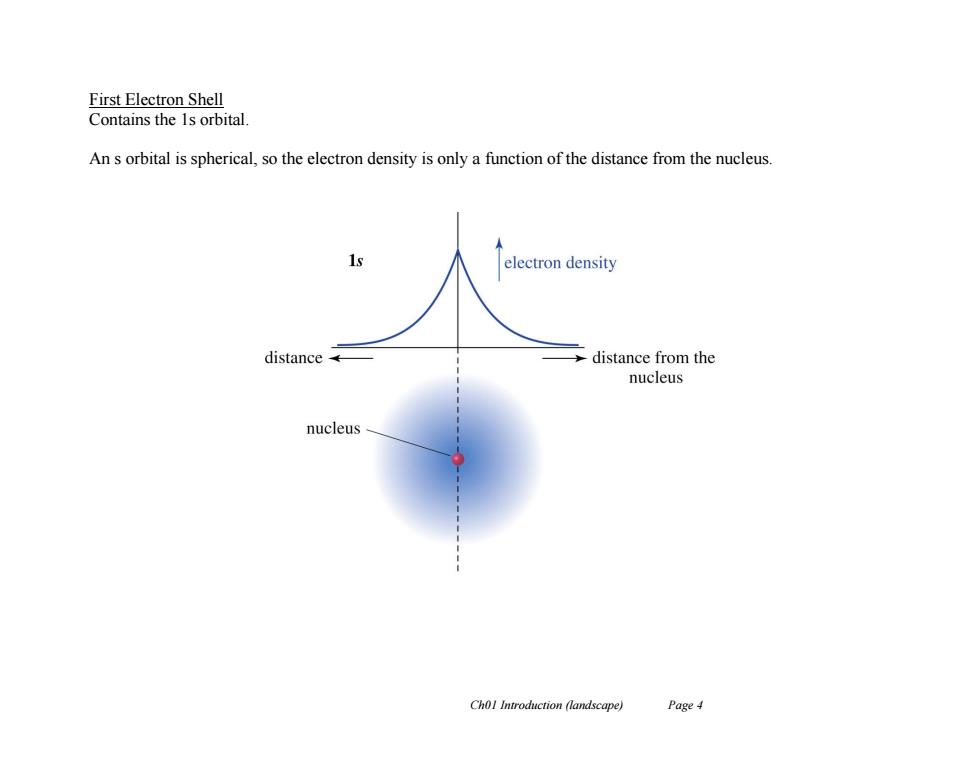
First Electron Shell Contains the 1s orbital. An s orbital is spherical,so the electron density is only a function of the distance from the nucleus. 1s electron density distance distance from the nucleus nucleus Chol Introduction (landscape) Page 4
Ch01 Introduction (landscape) Page 4 First Electron Shell Contains the 1s orbital. An s orbital is spherical, so the electron density is only a function of the distance from the nucleus
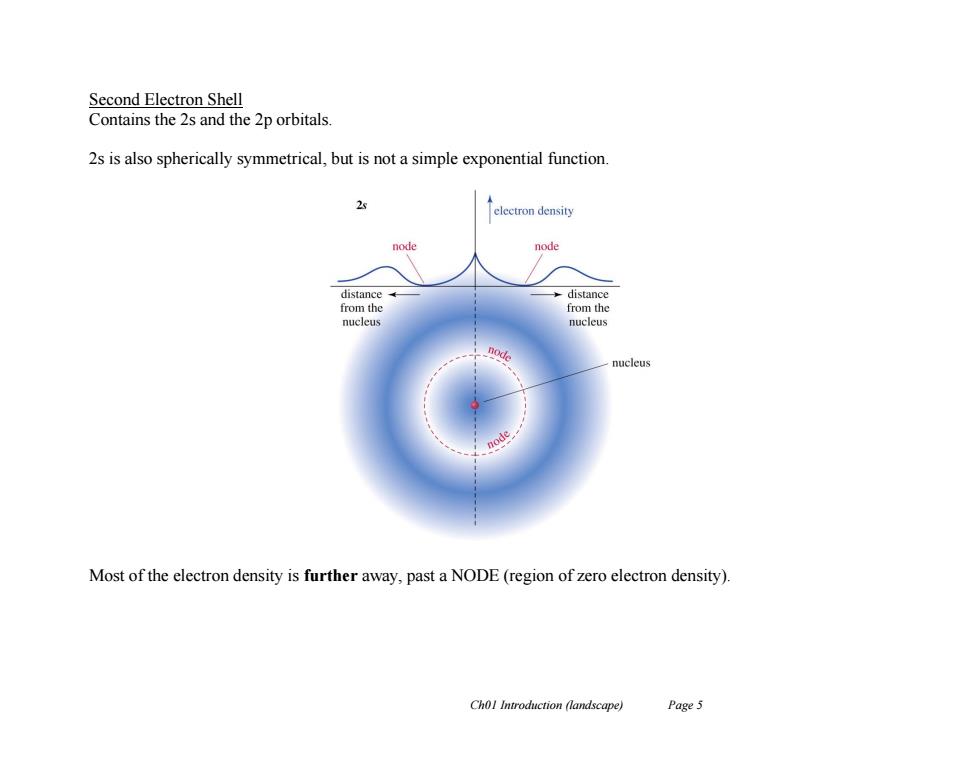
Second Electron Shell Contains the 2s and the 2p orbitals 2s is also spherically symmetrical,but is not a simple exponential function 2☒ electron density node node distance -→distance from the from the nucleu nucleus node nucleus node Most of the electron density is further away,past a NODE(region of zero electron density). Chol Introduction (landscape) Page 5
Ch01 Introduction (landscape) Page 5 Second Electron Shell Contains the 2s and the 2p orbitals. 2s is also spherically symmetrical, but is not a simple exponential function. Most of the electron density is further away, past a NODE (region of zero electron density)