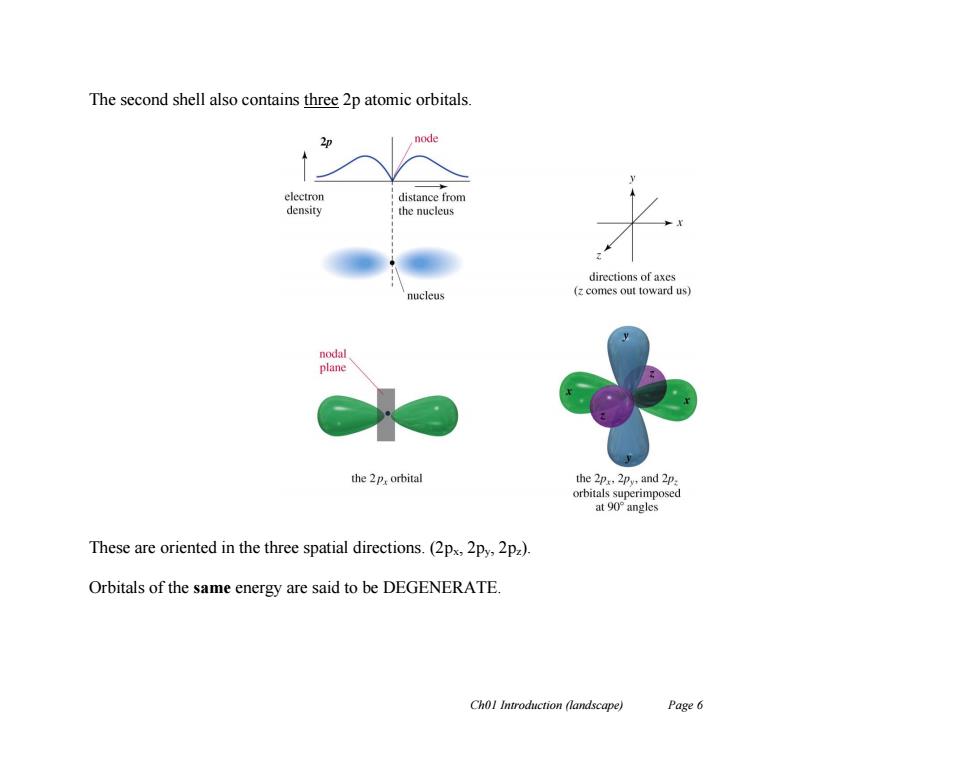
The second shell also contains three 2p atomic orbitals. 2p node electron distance from density the nucleus directions of axes nucleus (z comes out toward us) nodal plane the 2p,orbital the 2p 2py,and 2p. orbitals s rimposed at90°angles These are oriented in the three spatial directions.(2ps,2py,2pz). Orbitals of the same energy are said to be DEGENERATE. Chol Introduction (landscape) Page 6
Ch01 Introduction (landscape) Page 6 The second shell also contains three 2p atomic orbitals. These are oriented in the three spatial directions. (2px, 2py, 2pz). Orbitals of the same energy are said to be DEGENERATE
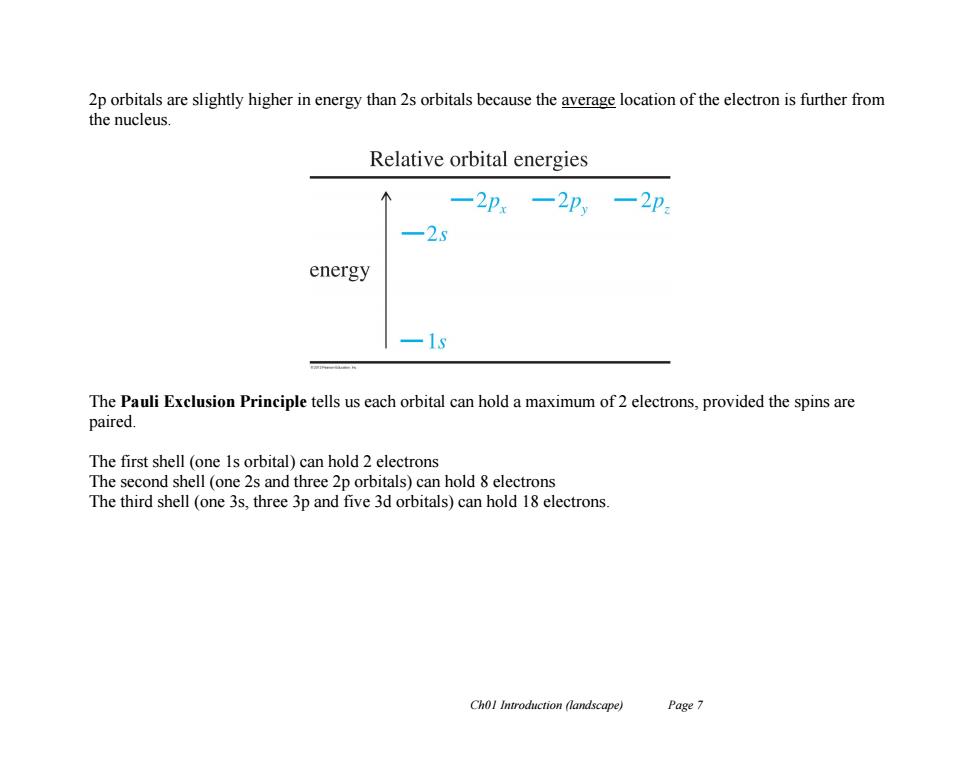
2p orbitals are slightly higher in energy than 2s orbitals because the average location of the electron is further from the nucleus. Relative orbital energies -2Px -2p,-2p -25 energy 一1 The Pauli Exclusion Principle tells us each orbital can hold a maximum of 2 electrons,provided the spins are paired. The first shell (one 1s orbital)can hold 2 electrons The second shell (one 2s and three 2p orbitals)can hold 8 electrons The third shell (one 3s.three 3p and five 3d orbitals)can hold 18 electrons. Chol Introduction (landscape) Page 7
Ch01 Introduction (landscape) Page 7 2p orbitals are slightly higher in energy than 2s orbitals because the average location of the electron is further from the nucleus. The Pauli Exclusion Principle tells us each orbital can hold a maximum of 2 electrons, provided the spins are paired. The first shell (one 1s orbital) can hold 2 electrons The second shell (one 2s and three 2p orbitals) can hold 8 electrons The third shell (one 3s, three 3p and five 3d orbitals) can hold 18 electrons
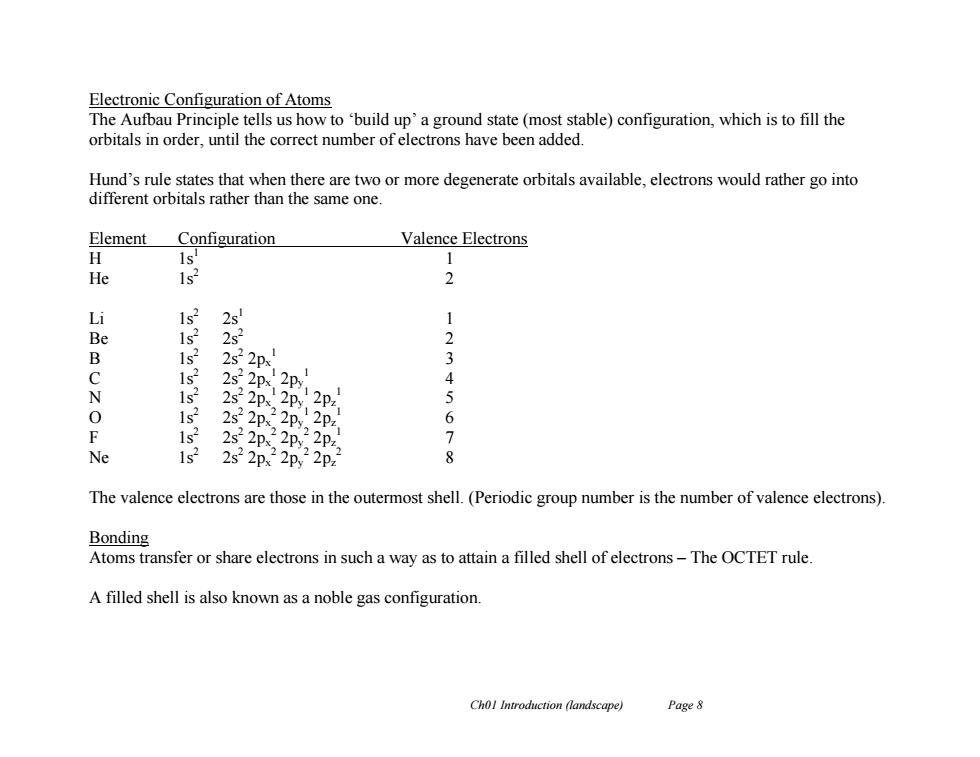
Electronic Configuration of Atoms The Aufbau Principle tells us how to 'build up'a ground state(most stable)configuration,which is to fill the orbitals in order,until the correct number of electrons have been added. Hund's rule states that when there are two or more degenerate orbitals available,electrons would rather go into different orbitals rather than the same one. Element Configuration Valence Electrons H ls' 1 He 2 Li 1s2 2 1 Be 1s 2s2 2 B 1s? 2s22px 3 1s 2s2 4 N 1s 2s2 5 0 1s? 2s2 2px 2py,2pz 6 F 1s 2s2 2px 2Py 7 Ne 1s2 2s22px22p,22p2 8 The valence electrons are those in the outermost shell.(Periodic group number is the number of valence electrons). Bonding Atoms transfer or share electrons in such a way as to attain a filled shell of electrons-The OCTET rule A filled shell is also known as a noble gas configuration. Chol Introduction (landscape) Page 8
Ch01 Introduction (landscape) Page 8 Electronic Configuration of Atoms The Aufbau Principle tells us how to ‘build up’ a ground state (most stable) configuration, which is to fill the orbitals in order, until the correct number of electrons have been added. Hund’s rule states that when there are two or more degenerate orbitals available, electrons would rather go into different orbitals rather than the same one. Element Configuration Valence Electrons H 1s1 1 He 1s2 2 Li 1s2 2s1 1 Be 1s2 2s2 2 B 1s2 2s2 2px 1 3 C 1s2 2s2 2px 1 2py 1 4 N 1s2 2s2 2px 1 2py 1 2pz 1 5 O 1s2 2s2 2px 2 2py 1 2pz 1 6 F 1s2 2s2 2px 2 2py 2 2pz 1 7 Ne 1s2 2s2 2px 2 2py 2 2pz 2 8 The valence electrons are those in the outermost shell. (Periodic group number is the number of valence electrons). Bonding Atoms transfer or share electrons in such a way as to attain a filled shell of electrons – The OCTET rule. A filled shell is also known as a noble gas configuration
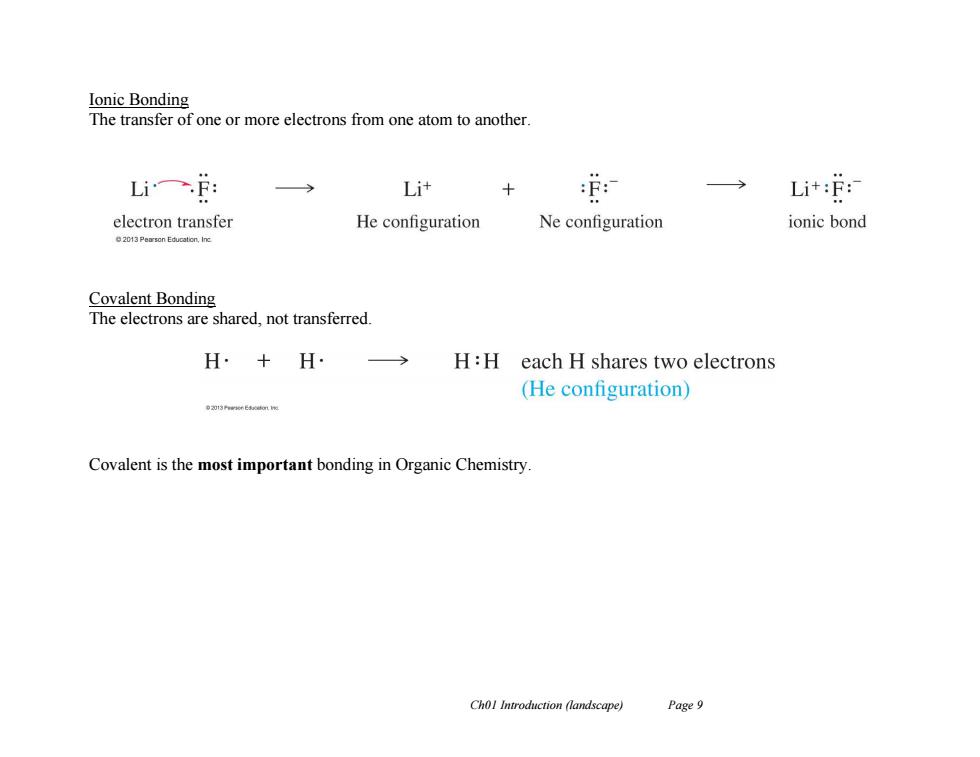
Ionic Bonding The transfer of one or more electrons from one atom to another Li一f: Li+ Li+:F: electron transfer He configuration Ne configuration ionic bond Covalent Bonding The electrons are shared,not transferred. H·+H H:H each H shares two electrons (He configuration) o动13 Covalent is the most important bonding in Organic Chemistry. Chol Introduction (landscape) Page 9
Ch01 Introduction (landscape) Page 9 Ionic Bonding The transfer of one or more electrons from one atom to another. Covalent Bonding The electrons are shared, not transferred. Covalent is the most important bonding in Organic Chemistry
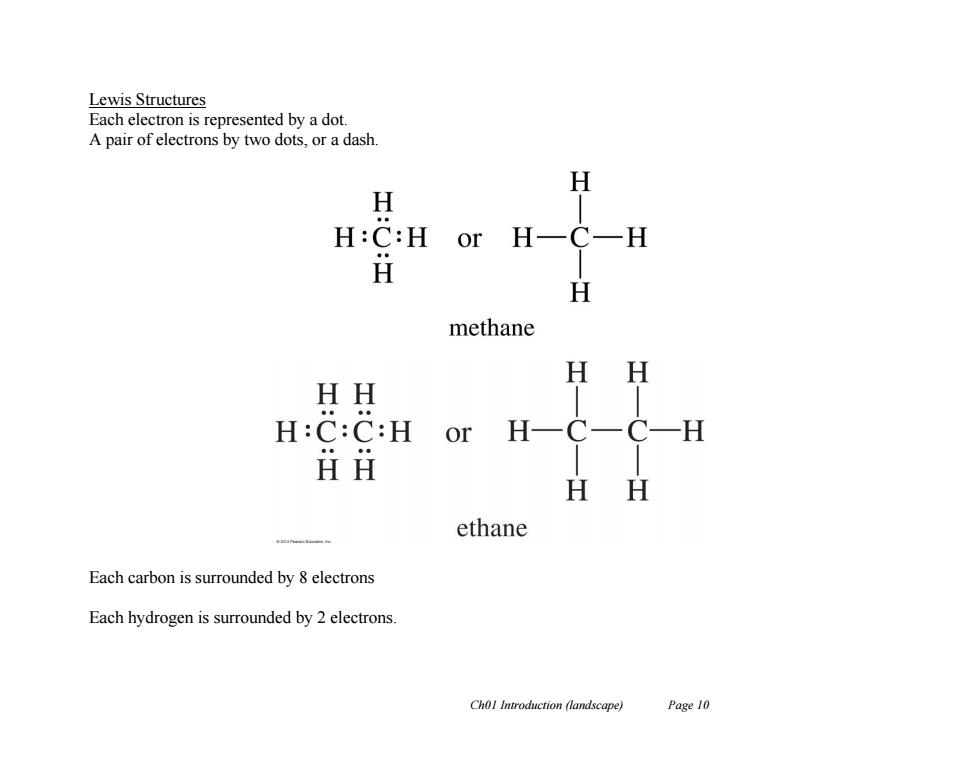
Lewis Structures Each electron is represented by a dot. A pair of electrons by two dots,or a dash. H H H:C:H or H H ⅱ H methane H H HH H:C:C:H or H一 HH H H ethane Each carbon is surrounded by 8 electrons Each hydrogen is surrounded by 2 electrons. Chol Introduction (landscape) Page 10
Ch01 Introduction (landscape) Page 10 Lewis Structures Each electron is represented by a dot. A pair of electrons by two dots, or a dash. Each carbon is surrounded by 8 electrons Each hydrogen is surrounded by 2 electrons