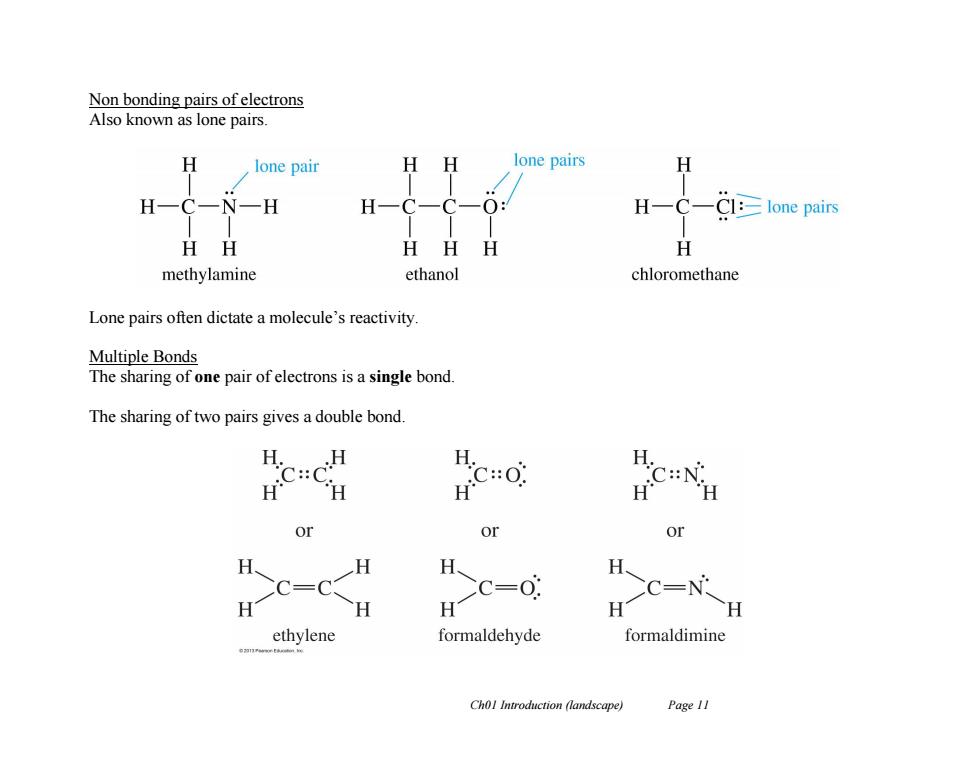
Non bonding pairs of electrons Also known as lone pairs. lone pair H lone pairs H H N一H H一C一 O: HC—Cl:≥lone pairs HH HHH 任 methylamine ethanol chloromethane Lone pairs often dictate a molecule's reactivity Multiple Bonds The sharing of one pair of electrons is a single bond. The sharing of two pairs gives a double bond. H.H H. C::C H.C:0 C:N: or or or H、 H H、 H CC=C C=0 CC=N H H H H H ethylene formaldehyde formaldimine Chol Introduction (landscape) Page 11
Ch01 Introduction (landscape) Page 11 Non bonding pairs of electrons Also known as lone pairs. Lone pairs often dictate a molecule’s reactivity. Multiple Bonds The sharing of one pair of electrons is a single bond. The sharing of two pairs gives a double bond
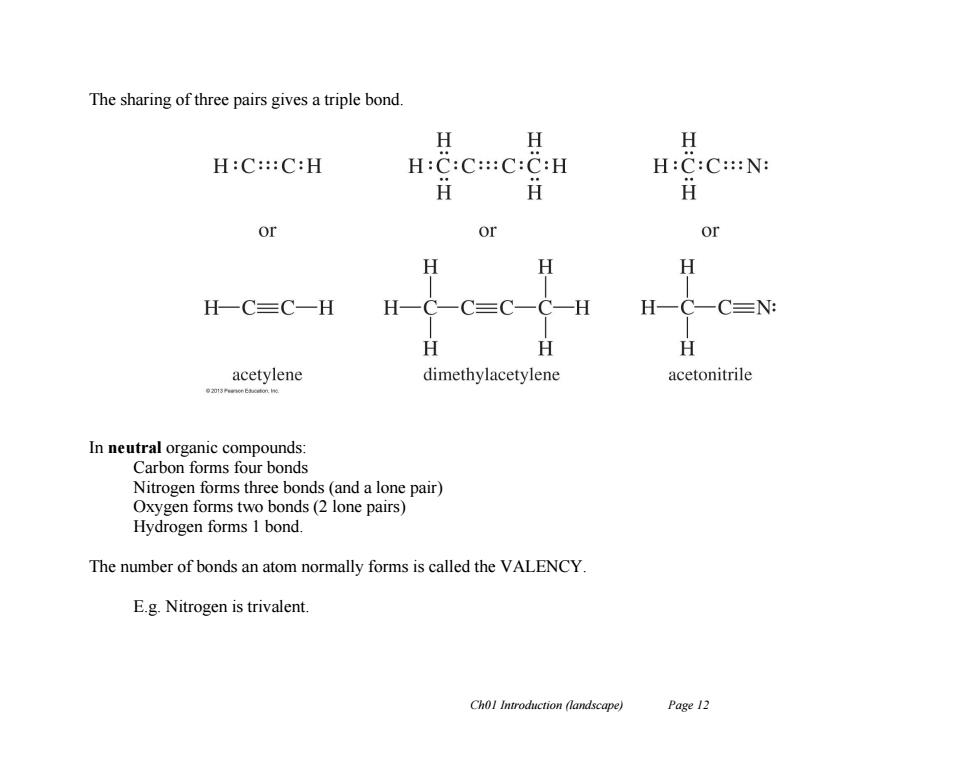
The sharing of three pairs gives a triple bond. H 女 H H:C:::C:H H:C:C:::C:C:H H:C:C::N: H or or or H H H H一C三C一H H -H H-C- CN: H H H acetylene dimethylacetylene acetonitrile In neutral organic compounds: Carbon forms four bonds Nitrogen forms three bonds(and a lone pair) Oxygen forms two bonds(2 lone pairs) Hydrogen forms 1 bond. The number of bonds an atom normally forms is called the VALENCY. E.g.Nitrogen is trivalent. Chol Introduction (landscape) Page 12
Ch01 Introduction (landscape) Page 12 The sharing of three pairs gives a triple bond. In neutral organic compounds: Carbon forms four bonds Nitrogen forms three bonds (and a lone pair) Oxygen forms two bonds (2 lone pairs) Hydrogen forms 1 bond. The number of bonds an atom normally forms is called the VALENCY. E.g. Nitrogen is trivalent