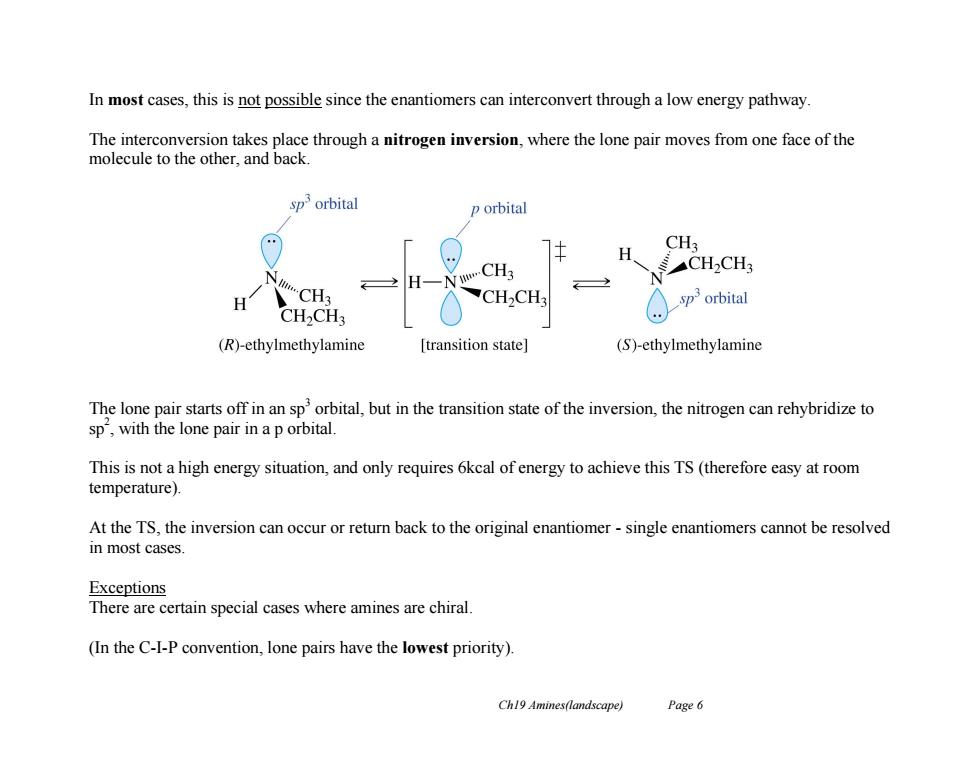
In most cases,this is not possible since the enantiomers can interconvert through a low energy pathway The interconversion takes place through a nitrogen inversion,where the lone pair moves from one face of the molecule to the other,and back sporbital p orbital CH3 CH2CH3 N H CH3 H CH3 CH2CH3 sporbital CH2CH3 (R)-ethylmethylamine [transition state] (S)-ethylmethylamine The lone pair starts off in an sp'orbital,but in the transition state of the inversion,the nitrogen can rehybridize to sp,with the lone pair in a p orbital. This is not a high energy situation,and only requires 6kcal of energy to achieve this TS(therefore easy at room temperature). At the TS,the inversion can occur or return back to the original enantiomer-single enantiomers cannot be resolved in most cases. Exceptions There are certain special cases where amines are chiral. (In the C-I-P convention,lone pairs have the lowest priority). Ch19 Amines(landscape) Page 6
Ch19 Amines(landscape) Page 6 In most cases, this is not possible since the enantiomers can interconvert through a low energy pathway. The interconversion takes place through a nitrogen inversion, where the lone pair moves from one face of the molecule to the other, and back. The lone pair starts off in an sp3 orbital, but in the transition state of the inversion, the nitrogen can rehybridize to sp 2 , with the lone pair in a p orbital. This is not a high energy situation, and only requires 6kcal of energy to achieve this TS (therefore easy at room temperature). At the TS, the inversion can occur or return back to the original enantiomer - single enantiomers cannot be resolved in most cases. Exceptions There are certain special cases where amines are chiral. (In the C-I-P convention, lone pairs have the lowest priority)
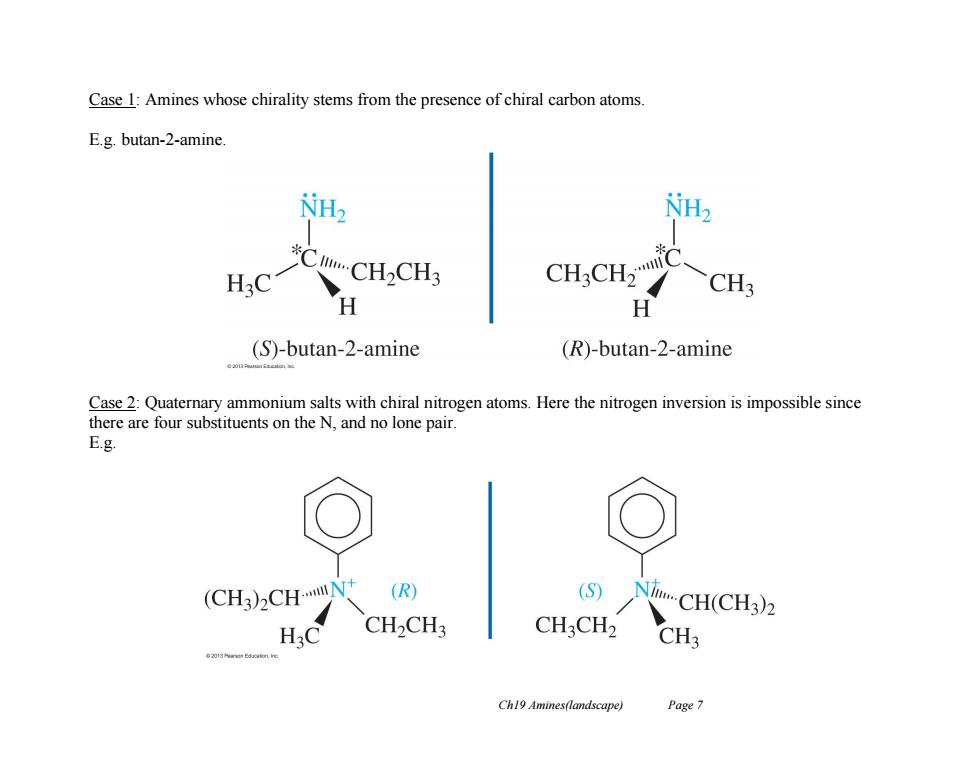
Case 1:Amines whose chirality stems from the presence of chiral carbon atoms. E.g.butan-2-amine. CH.CH CH3 H (S)-butan-2-amine (R)-butan-2-amine 020 Case 2:Quaternary ammonium salts with chiral nitrogen atoms.Here the nitrogen inversion is impossible since there are four substituents on the N,and no lone pair. E.g. (CH3)2CHN (R) (S) NiCH(CH3)2 CH2CH3 CHCH2 CH3 Ch19 Amines(landscape) Page 7
Ch19 Amines(landscape) Page 7 Case 1: Amines whose chirality stems from the presence of chiral carbon atoms. E.g. butan-2-amine. Case 2: Quaternary ammonium salts with chiral nitrogen atoms. Here the nitrogen inversion is impossible since there are four substituents on the N, and no lone pair. E.g
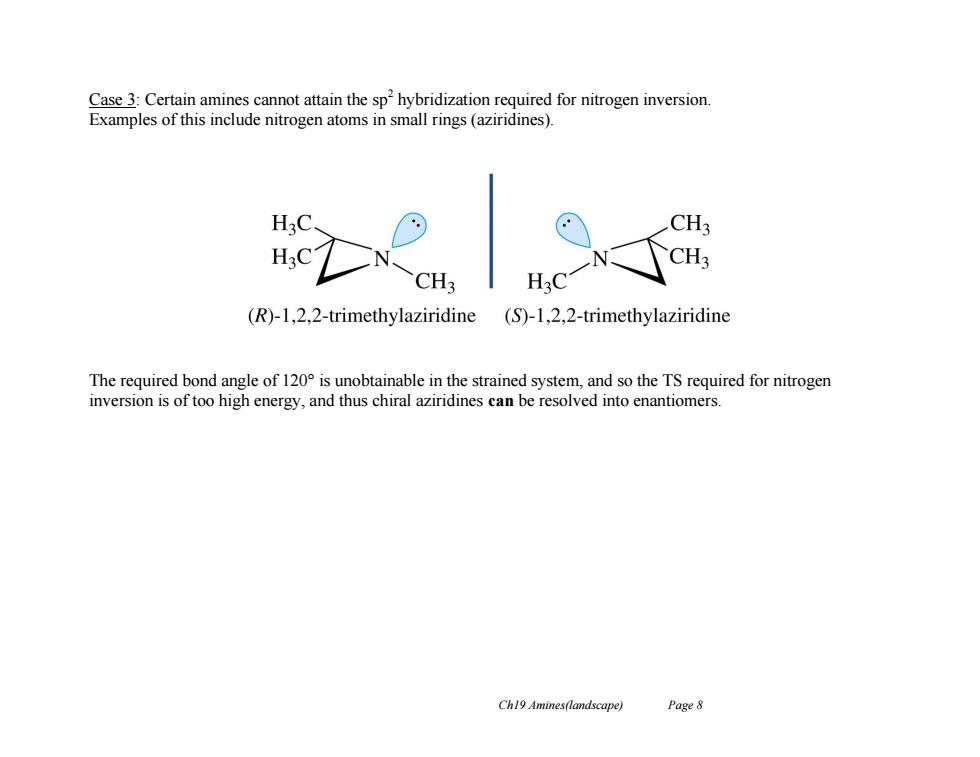
Case 3:Certain amines cannot attain the sp'hybridization required for nitrogen inversion. Examples of this include nitrogen atoms in small rings(aziridines). H3 CH; H30 CH; CH3 H3C (R)-1,2,2-trimethylaziridine (S)-1,2,2-trimethylaziridine The required bond angle of 120 is unobtainable in the strained system,and so the TS required for nitrogen inversion is of too high energy,and thus chiral aziridines can be resolved into enantiomers. Ch19 Amines(landscape) Page 8
Ch19 Amines(landscape) Page 8 Case 3: Certain amines cannot attain the sp2 hybridization required for nitrogen inversion. Examples of this include nitrogen atoms in small rings (aziridines). The required bond angle of 120° is unobtainable in the strained system, and so the TS required for nitrogen inversion is of too high energy, and thus chiral aziridines can be resolved into enantiomers
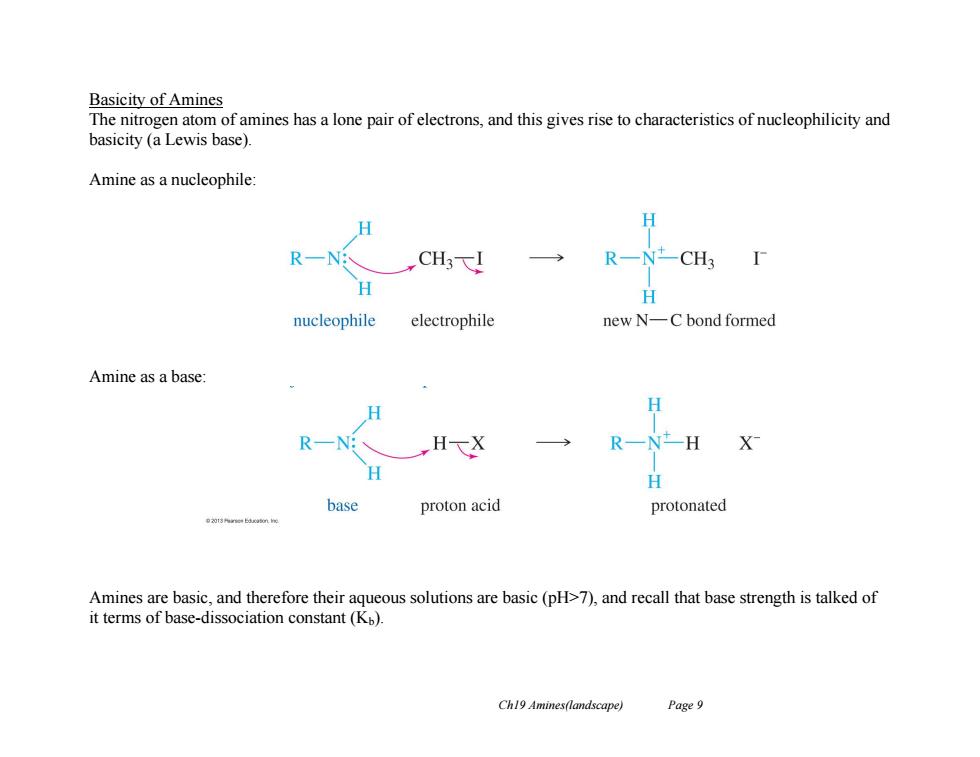
Basicity of Amines The nitrogen atom of amines has a lone pair of electrons,and this gives rise to characteristics of nucleophilicity and basicity (a Lewis base). Amine as a nucleophile H H R一NCH3F H nucleophile electrophile new N-C bond formed Amine as a base: H R一Nt- H X H base proton acid protonated Amines are basic,and therefore their aqueous solutions are basic (pH>7),and recall that base strength is talked of it terms of base-dissociation constant(Kp). Ch19 Amines(landscape) Page 9
Ch19 Amines(landscape) Page 9 Basicity of Amines The nitrogen atom of amines has a lone pair of electrons, and this gives rise to characteristics of nucleophilicity and basicity (a Lewis base). Amine as a nucleophile: Amine as a base: Amines are basic, and therefore their aqueous solutions are basic (pH>7), and recall that base strength is talked of it terms of base-dissociation constant (Kb)
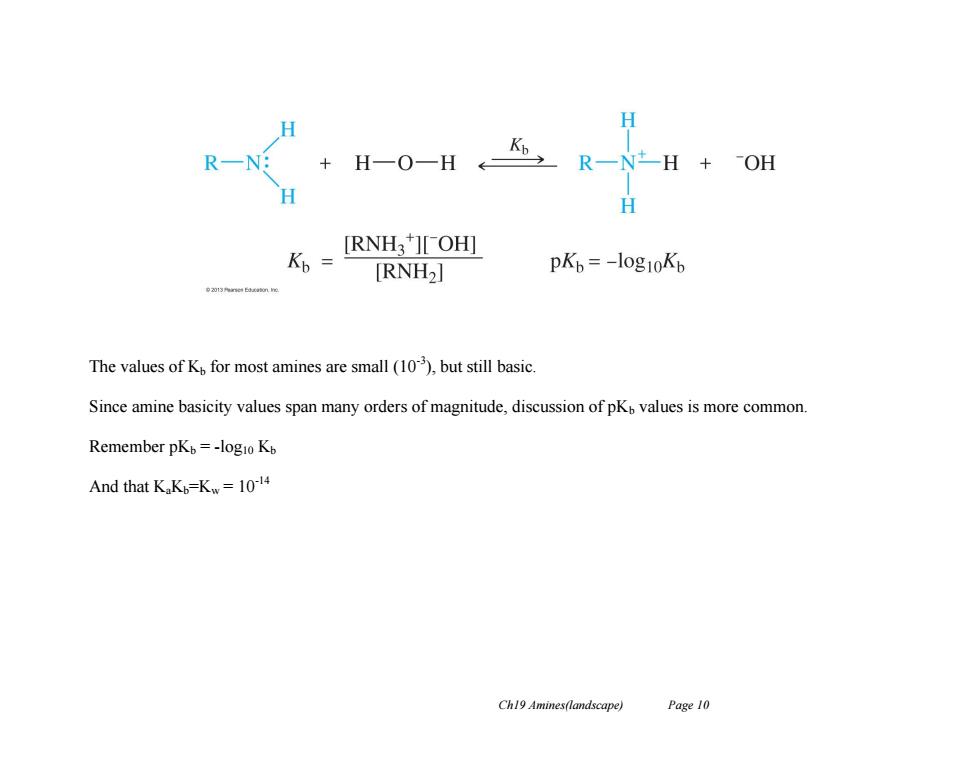
H R-N: H-0-HR-H+0附 + H K,= [RNH3][OH] [RNH2] pKb=-l0g10Kb The values of K for most amines are small (10),but still basic. Since amine basicity values span many orders of magnitude,discussion of pKo values is more common. Remember pKb=-logio Kb And that KaK=Kw=10-14 Ch19 Amines(landscape) Page 10
Ch19 Amines(landscape) Page 10 The values of Kb for most amines are small (10-3 ), but still basic. Since amine basicity values span many orders of magnitude, discussion of pKb values is more common. Remember pKb = -log10 Kb And that KaKb=Kw = 10-14