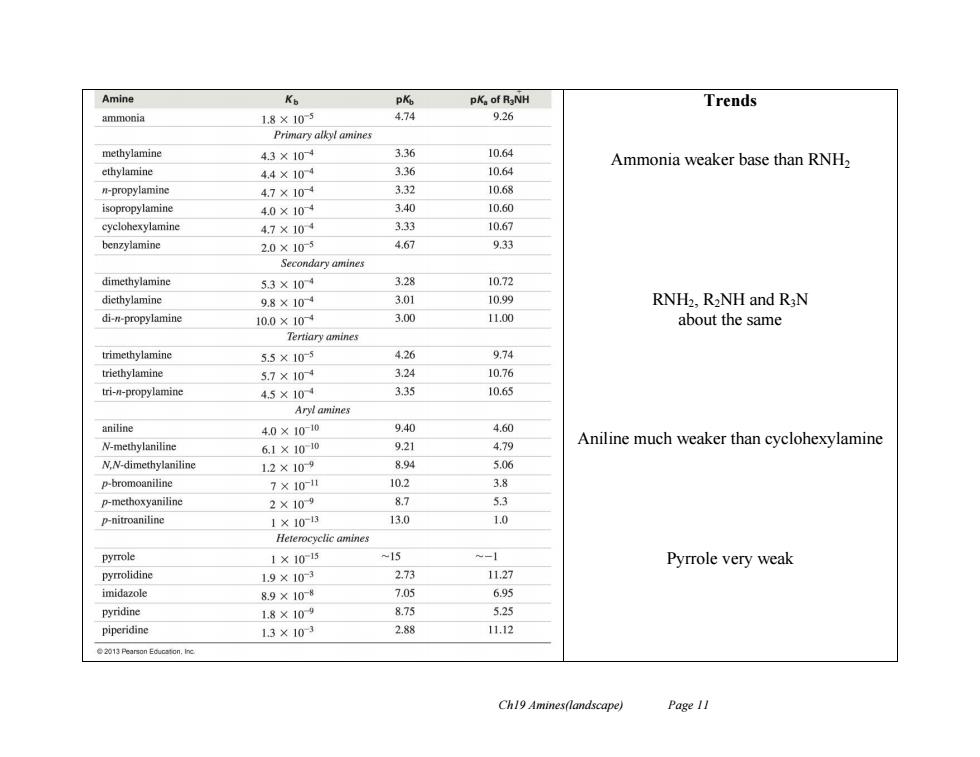
Amine Ko P飞 pK。of RaNH Trends ammonia 1.8×105 4.74 9.26 Primary alkyl amines methylamine 4.3×104 3.36 10.64 Ammonia weaker base than RNH2 ethylamine 4.4×104 3.36 10.64 n-propylamine 4.7×104 3.32 10.68 isopropylamine 4.0×10-4 3.40 10.60 cyclohexylamine 4.7×104 3.33 10.67 benzylamine 2.0×105 4.67 9.33 Secondary amines dimethylamine 5.3×104 3.28 10.72 diethylamine 9.8×104 3.01 10.99 RNH2,R2NH and R3N di-n-propylamine 10.0×10 3.00 11.00 about the same Tertiary amines trimethylamine 5.5×105 4.26 9.74 triethylamine 5.7×104 3.24 10.76 tri-n-propylamine 4.5×104 3.35 10.65 Aryl amines aniline 4.0×100 9.40 4.60 N-methylaniline 6.1×10-10 9.21 4.79 Aniline much weaker than cyclohexylamine N.N-dimethylaniline 1.2×109 8.94 5.06 p-bromoaniline 7×10-1 10.2 3.8 p-methoxyaniline 2×109 8.7 5.3 p-nitroaniline 1×103 13.0 1.0 Heterocyclic amines pyrrole 1×1015 -15 -1 Pyrrole very weak pyrrolidine 1.9×103 2.73 11.27 imidazole 8.9×108 7.05 6.95 pyridine 1.8×109 875 5.25 piperidine 13×103 2.88 11.12 Ch19 Amines(landscape) Page 11
Ch19 Amines(landscape) Page 11 Trends Ammonia weaker base than RNH2 RNH2, R2NH and R3N about the same Aniline much weaker than cyclohexylamine Pyrrole very weak
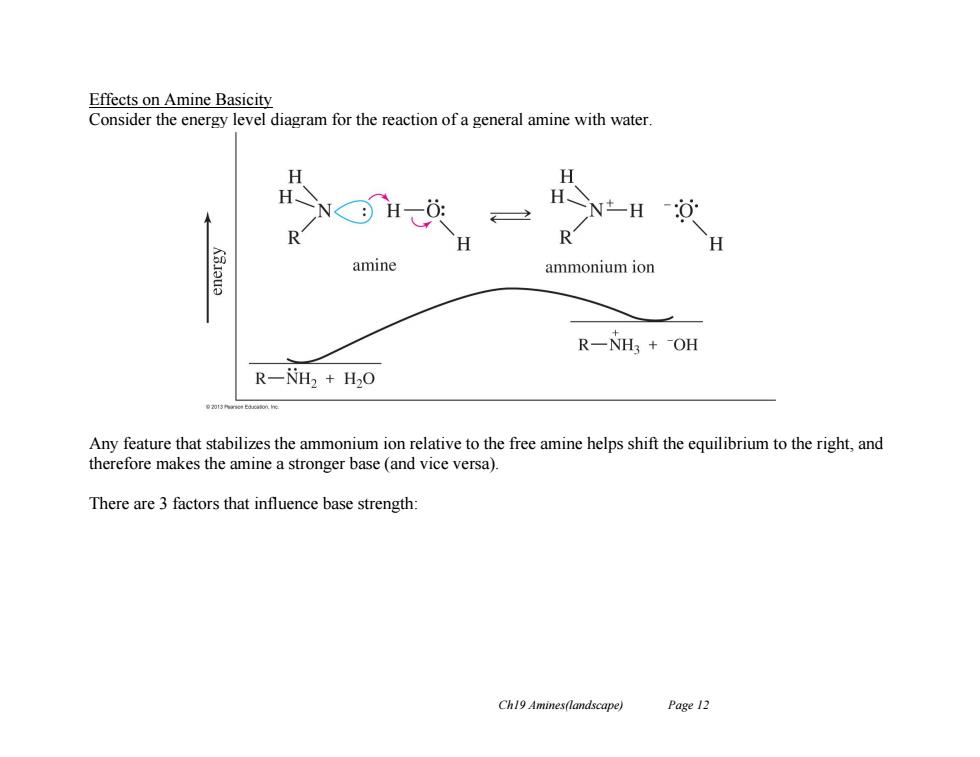
Effects on Amine Basicity Consider the energy level diagram for the reaction of a general amine with water H≥N amine ammonium ion R一NH3+-OH R-NH2 H2O Any feature that stabilizes the ammonium ion relative to the free amine helps shift the equilibrium to the right,and therefore makes the amine a stronger base (and vice versa). There are 3 factors that influence base strength: Ch19 Amines(landscape) Page 12
Ch19 Amines(landscape) Page 12 Effects on Amine Basicity Consider the energy level diagram for the reaction of a general amine with water. Any feature that stabilizes the ammonium ion relative to the free amine helps shift the equilibrium to the right, and therefore makes the amine a stronger base (and vice versa). There are 3 factors that influence base strength:
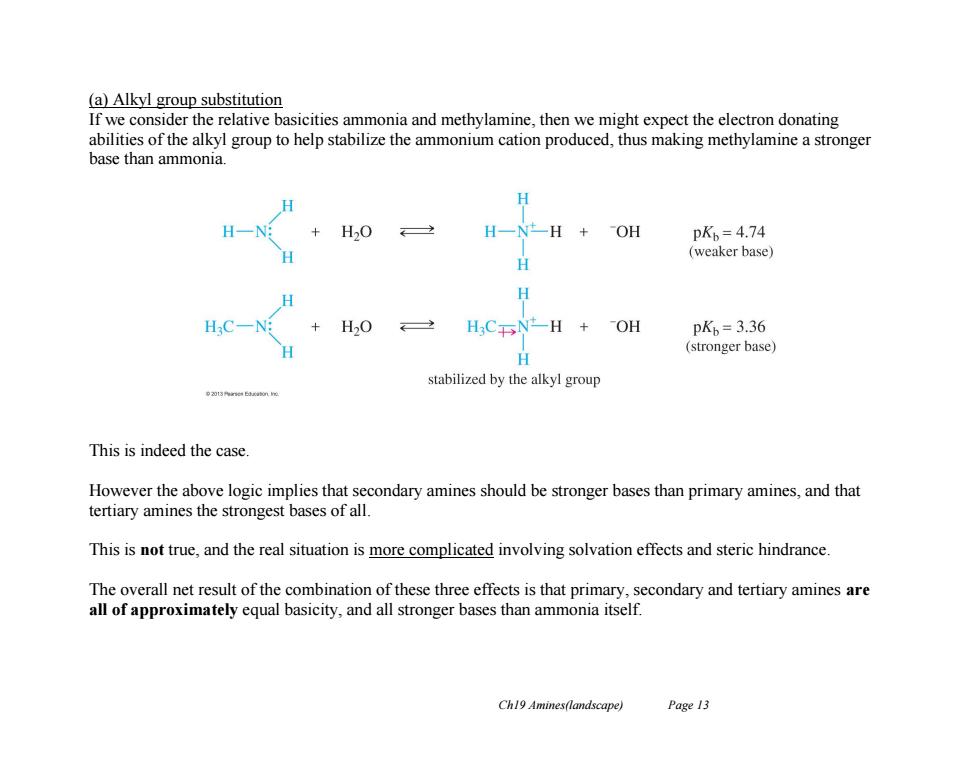
(a)Alkyl group substitution If we consider the relative basicities ammonia and methylamine,then we might expect the electron donating abilities of the alkyl group to help stabilize the ammonium cation produced,thus making methylamine a stronger base than ammonia. H H-N H一N一H+OH pKb=4.74 (weaker base) H HC一N +H20 2 H3CNH +OH pK=3.36 (stronger base) 日 stabilized by the alkyl group This is indeed the case. However the above logic implies that secondary amines should be stronger bases than primary amines,and that tertiary amines the strongest bases of all. This is not true,and the real situation is more complicated involving solvation effects and steric hindrance. The overall net result of the combination of these three effects is that primary,secondary and tertiary amines are all of approximately equal basicity,and all stronger bases than ammonia itself. Ch19 Amines(landscape) Page 13
Ch19 Amines(landscape) Page 13 (a) Alkyl group substitution If we consider the relative basicities ammonia and methylamine, then we might expect the electron donating abilities of the alkyl group to help stabilize the ammonium cation produced, thus making methylamine a stronger base than ammonia. This is indeed the case. However the above logic implies that secondary amines should be stronger bases than primary amines, and that tertiary amines the strongest bases of all. This is not true, and the real situation is more complicated involving solvation effects and steric hindrance. The overall net result of the combination of these three effects is that primary, secondary and tertiary amines are all of approximately equal basicity, and all stronger bases than ammonia itself
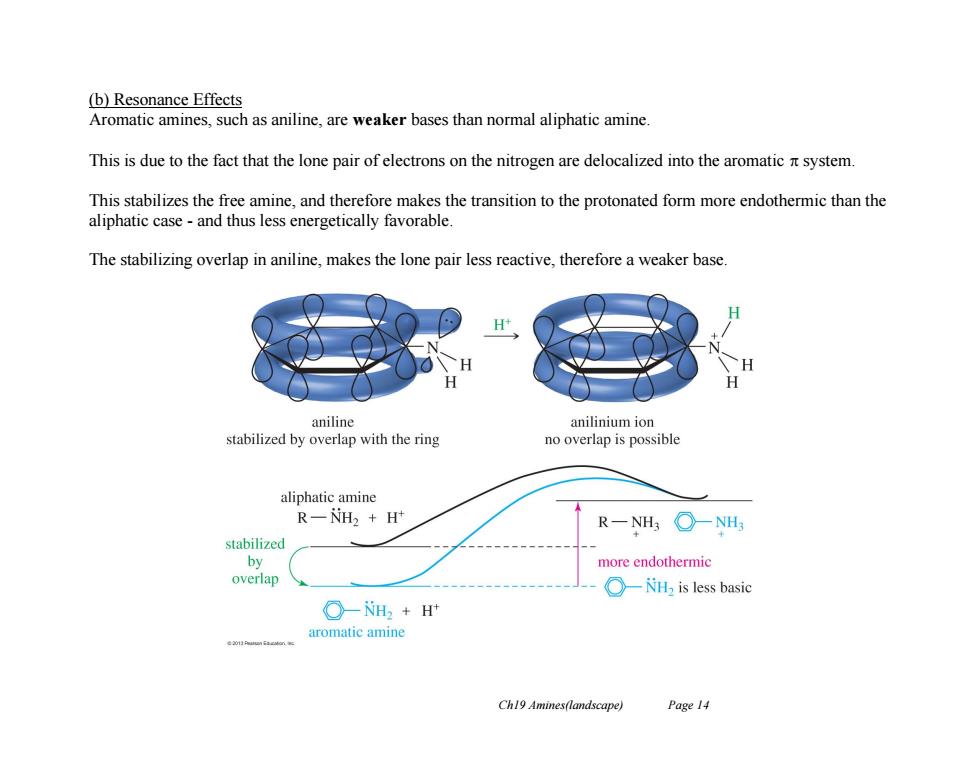
(b)Resonance Effects Aromatic amines,such as aniline,are weaker bases than normal aliphatic amine This is due to the fact that the lone pair of electrons on the nitrogen are delocalized into the aromatic system. This stabilizes the free amine,and therefore makes the transition to the protonated form more endothermic than the aliphatic case-and thus less energetically favorable. The stabilizing overlap in aniline,makes the lone pair less reactive,therefore a weaker base. H aniline anilinium ion stabilized by overlap with the ring no overlap is possible aliphatic amine R一NH2+H R-NH3○-NH stabilized by more endothermic overlap ○-il2 is less basic ○-NH2+H aromatic amine Ch19 Amines(landscape) Page 14
Ch19 Amines(landscape) Page 14 (b) Resonance Effects Aromatic amines, such as aniline, are weaker bases than normal aliphatic amine. This is due to the fact that the lone pair of electrons on the nitrogen are delocalized into the aromatic system. This stabilizes the free amine, and therefore makes the transition to the protonated form more endothermic than the aliphatic case - and thus less energetically favorable. The stabilizing overlap in aniline, makes the lone pair less reactive, therefore a weaker base
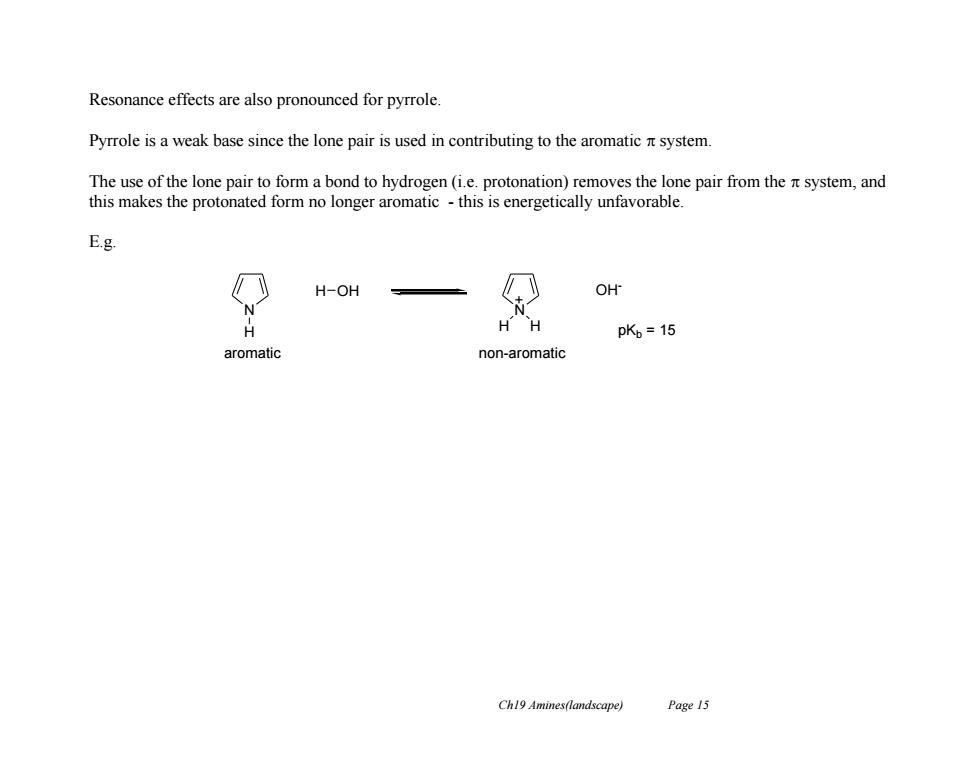
Resonance effects are also pronounced for pyrrole. Pyrrole is a weak base since the lone pair is used in contributing to the aromatic nt system The use of the lone pair to form a bond to hydrogen(i.e.protonation)removes the lone pair from the system,and this makes the protonated form no longer aromatic-this is energetically unfavorable. E.g. H-OH OH H HH pK,=15 aromatic non-aromatic Ch19 Amines(landscape) Page 15
Ch19 Amines(landscape) Page 15 Resonance effects are also pronounced for pyrrole. Pyrrole is a weak base since the lone pair is used in contributing to the aromatic system. The use of the lone pair to form a bond to hydrogen (i.e. protonation) removes the lone pair from the system, and this makes the protonated form no longer aromatic - this is energetically unfavorable. E.g. N H H OH N OH - H H pKb = 15 + aromatic non-aromatic