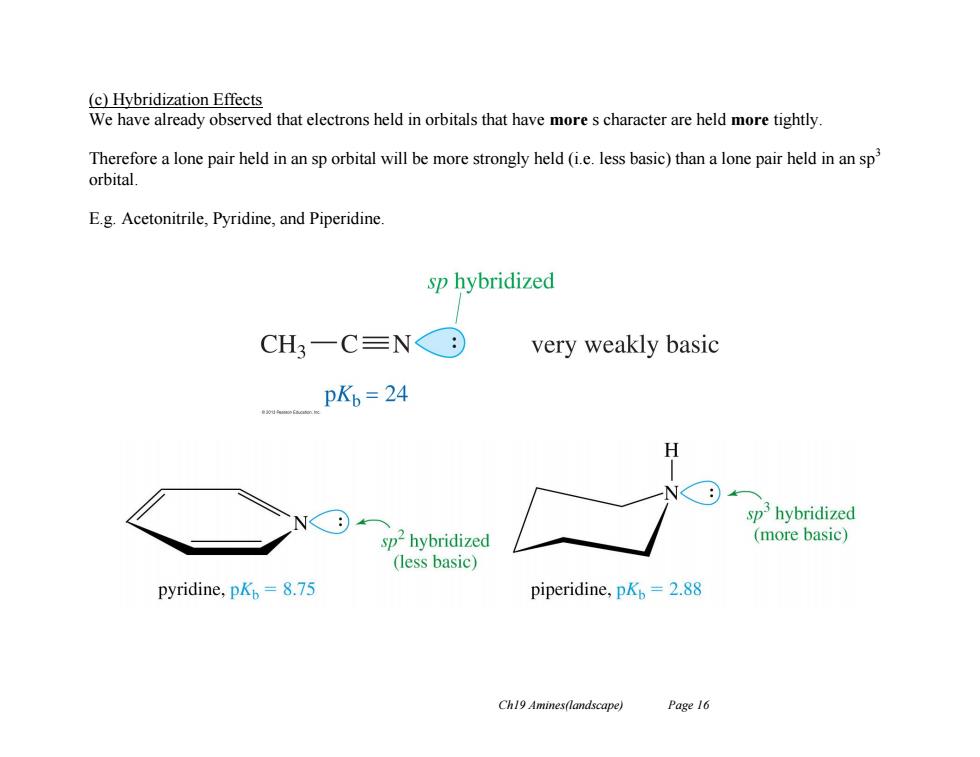
(c)Hybridization Effects We have already observed that electrons held in orbitals that have more s character are held more tightly Therefore a lone pair held in an sp orbital will be more strongly held(i.e.less basic)than a lone pair held in an sp orbital. E.g.Acetonitrile,Pyridine,and Piperidine. sp hybridized CH3-C≡N⊙ very weakly basic pKb=24 H sp'hybridized sp2hybridized (more basic) (less basic) pyridine,pKp=8.75 piperidine,pKp=2.88 Ch19 Amines(landscape) Page 16
Ch19 Amines(landscape) Page 16 (c) Hybridization Effects We have already observed that electrons held in orbitals that have more s character are held more tightly. Therefore a lone pair held in an sp orbital will be more strongly held (i.e. less basic) than a lone pair held in an sp3 orbital. E.g. Acetonitrile, Pyridine, and Piperidine
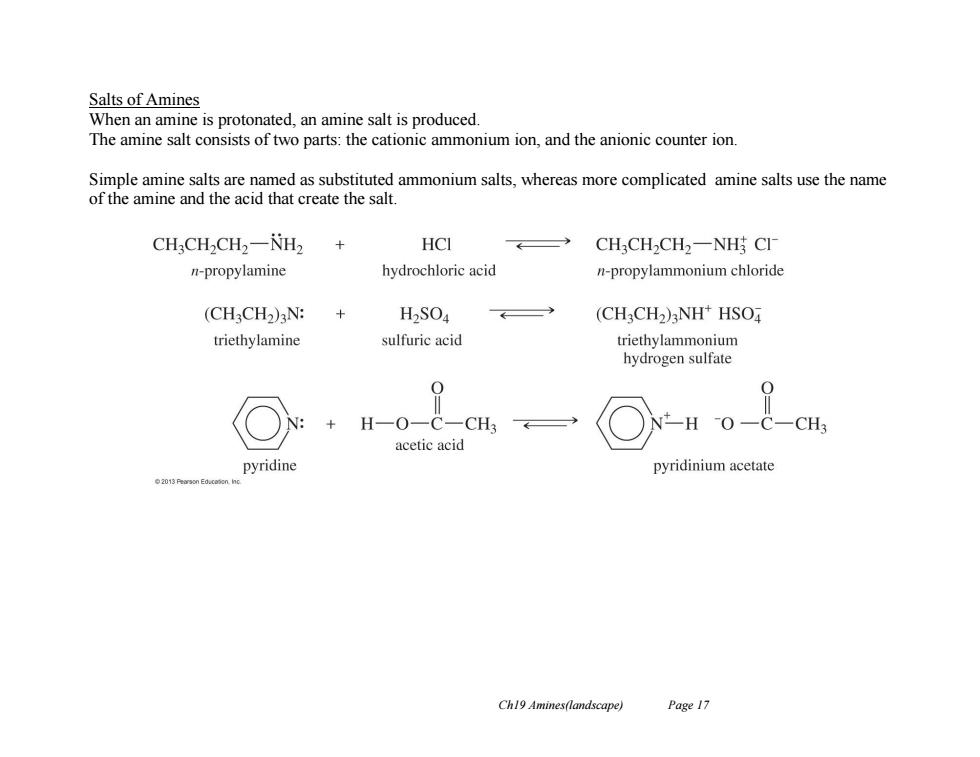
Salts of Amines When an amine is protonated,an amine salt is produced. The amine salt consists of two parts:the cationic ammonium ion,and the anionic counter ion. Simple amine salts are named as substituted ammonium salts,whereas more complicated amine salts use the name of the amine and the acid that create the salt. CH:CH2CH2-NH2 HCI CH3CH2CH2一NH CI n-propylamine hydrochloric acid n-propylammonium chloride (CHCH2)N: + H2SO4 (CH3CH2)3NH*HSO triethylamine sulfuric acid triethylammonium hydrogen sulfate 0 H一O一C一CH3 -HO -C-CH3 acetic acid pyridine pyridinium acetate Ch19 Amines(landscape) Page 17
Ch19 Amines(landscape) Page 17 Salts of Amines When an amine is protonated, an amine salt is produced. The amine salt consists of two parts: the cationic ammonium ion, and the anionic counter ion. Simple amine salts are named as substituted ammonium salts, whereas more complicated amine salts use the name of the amine and the acid that create the salt

Amines are generally volatile,smelly liquids,whereas the ammonium salts are crystalline,high melting solids These ionic solids are soluble in water,but insoluble in organic solvents The free amines are generally insoluble in water,but soluble in organic solvents. This provides an excellent method for the separation and isolation of amine compounds. Free amines are insoluble in water,but when dilute acid is added,the ammonium salt is produced,which dissolves. aq.HCI RN: RNH CI “free'amine aq.NaOH amine salt (water insoluble) (water soluble) When the solution is made alkaline(by adding NaOH),the now purified free amine is regenerated,which is insoluble in the aqueous solution and therefore precipitates,or can be extracted into an organic solvent. Ch19 Amines(landscape) Page 18
Ch19 Amines(landscape) Page 18 Amines are generally volatile, smelly liquids, whereas the ammonium salts are crystalline, high melting solids. These ionic solids are soluble in water, but insoluble in organic solvents. The free amines are generally insoluble in water, but soluble in organic solvents. This provides an excellent method for the separation and isolation of amine compounds. Free amines are insoluble in water, but when dilute acid is added, the ammonium salt is produced, which dissolves. When the solution is made alkaline (by adding NaOH), the now purified free amine is regenerated, which is insoluble in the aqueous solution and therefore precipitates, or can be extracted into an organic solvent

ether RaN: rganics RaN: phase other (1)remove ether phase (pure) organics (1)remove H2O phase (2)add NaOH (2)add dilute HCL HCI (3)add fresh ether H2O salts. RNH CI phase ete. NaoH RN: HCI →RNHC1 NaOH RN: soluble in ether insoluble in ether soluble in ether insoluble in H2O soluble in H3O insoluble in HO amine and shake with organic H and H2O shake with cther amine mixture ether/water ac amineOH.ethe Wale之inorganie impurities and salts This procedure is typical/useful for the purification of all amine containing compounds. E.g.powder cocaine(snort) crack (smoke). CH3 CH3 0 CI NaOH -OCH; or NaHCO3 OCH 0 HCI H cocaine hydrochloride cocaine“free base'" Ch19 Amines(landscape) Page 19
Ch19 Amines(landscape) Page 19 This procedure is typical/useful for the purification of all amine containing compounds. E.g. powder cocaine (snort) crack (smoke)

Reactions of Amines With Carbonyl Groups We have already seen the reaction of various amines with ketones and aldehydes to generate imines and their analogues. E.g. H + H20 R R R R R ketone or aldehyde carbinolamine derivative Y=H or alkyl gives an imine Y=OH gives an oxime Y =NHR gives a hydrazone Ch19 Amines(landscape) Page 20
Ch19 Amines(landscape) Page 20 Reactions of Amines With Carbonyl Groups We have already seen the reaction of various amines with ketones and aldehydes to generate imines and their analogues. E.g