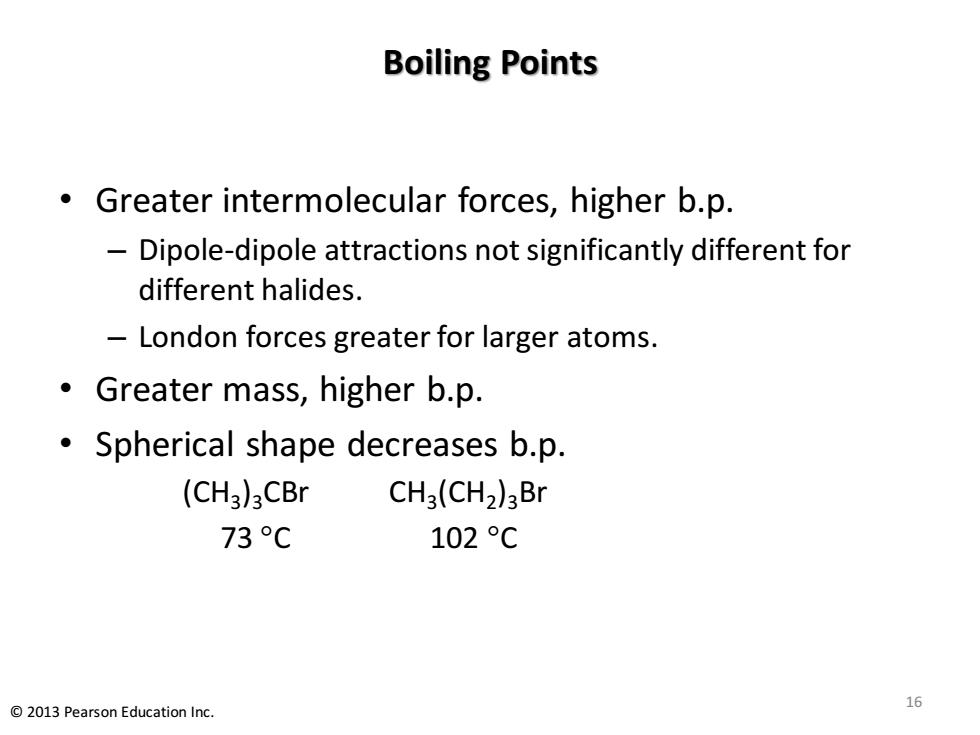
Boiling Points Greater intermolecular forces,higher b.p. Dipole-dipole attractions not significantly different for different halides. London forces greater for larger atoms. Greater mass,higher b.p. Spherical shape decreases b.p. (CH3)3CBr CH3(CH2)3Br 73C 102℃ 2013 Pearson Education Inc. 16
Boiling Points • Greater intermolecular forces, higher b.p. – Dipole-dipole attractions not significantly different for different halides. – London forces greater for larger atoms. • Greater mass, higher b.p. • Spherical shape decreases b.p. (CH3 )3CBr CH3 (CH2 )3Br 73 C 102 C 16 © 2013 Pearson Education Inc

Densities Alkyl fluorides and alkyl chlorides (those with just one chlorine atom)are less dense than water (1.00g/ml). Alkyl chlorides with two or more chlorine atoms are denser than water. All alkyl bromides and alkyl iodides are denser than water. 2013 Pearson Education Inc
Densities • Alkyl fluorides and alkyl chlorides (those with just one chlorine atom) are less dense than water (1.00 g/mL). • Alkyl chlorides with two or more chlorine atoms are denser than water. • All alkyl bromides and alkyl iodides are denser than water. 17 © 2013 Pearson Education Inc

Preparation of Alkyl Halides Free radical halogenation (Chapter 4) -Chlorination produces a mixtures of products. This reaction is not a good lab synthesis,except in alkanes where all hydrogens are equivalent. Bromination is highly selective. Free radical allylic halogenation Halogen is placed on a carbon directly attached to the double bond (allylic). 2013 Pearson Education Inc. 18
Preparation of Alkyl Halides • Free radical halogenation (Chapter 4) – Chlorination produces a mixtures of products. This reaction is not a good lab synthesis, except in alkanes where all hydrogens are equivalent. – Bromination is highly selective. • Free radical allylic halogenation – Halogen is placed on a carbon directly attached to the double bond (allylic). 18 © 2013 Pearson Education Inc
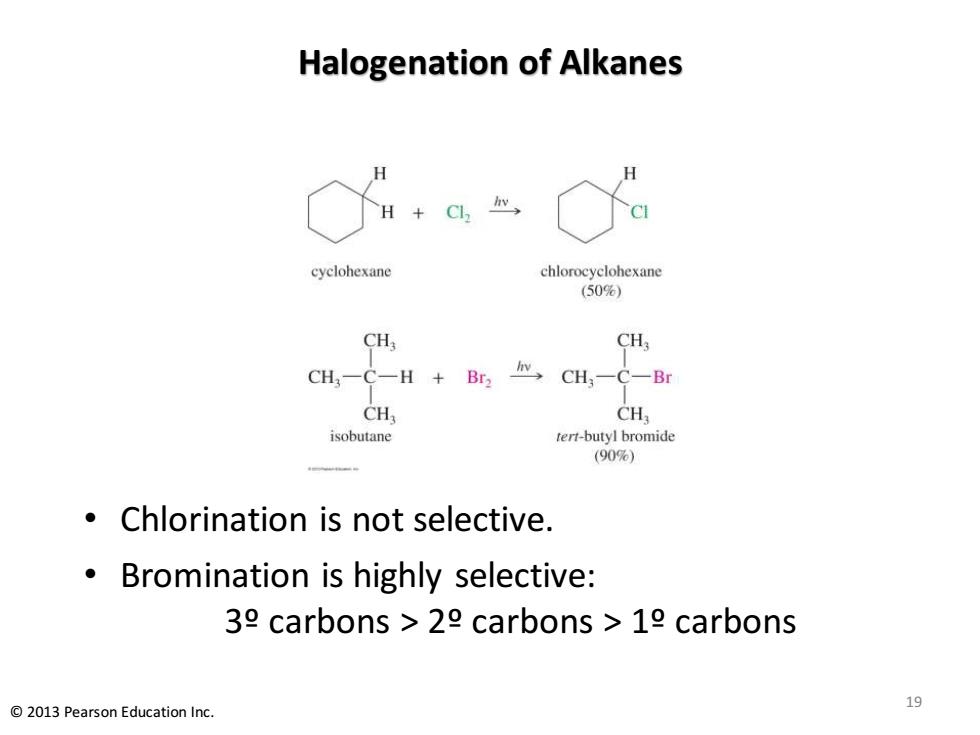
Halogenation of Alkanes +C, H cyclohexane chlorocyclohexane (50%) CHs CH CH一C一H+ Br2 CH-C-Br CH, CH isobutane tert-butyl bromide (90%) Chlorination is not selective. Bromination is highly selective: 39 carbons 29 carbons 19 carbons 2013 Pearson Education Inc. 19
Halogenation of Alkanes • Chlorination is not selective. • Bromination is highly selective: 3º carbons > 2º carbons > 1º carbons 19 © 2013 Pearson Education Inc
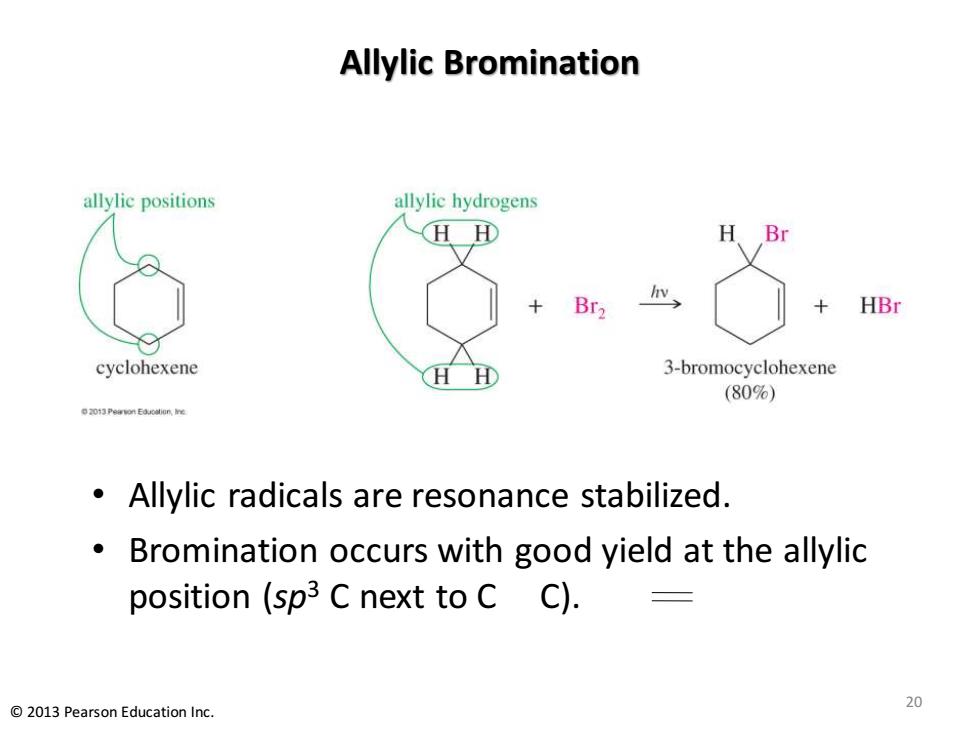
Allylic Bromination allylic positions allylic hydrogens H田 H Br Br2 HBr cyclohexene H 3-bromocyclohexene (80%) 2013Peavon Eouoalene Allylic radicals are resonance stabilized. Bromination occurs with good yield at the allylic position (sp3 C next to cC). 2013 Pearson Education Inc. 20
Allylic Bromination • Allylic radicals are resonance stabilized. • Bromination occurs with good yield at the allylic position (sp3 C next to C C). 20 © 2013 Pearson Education Inc