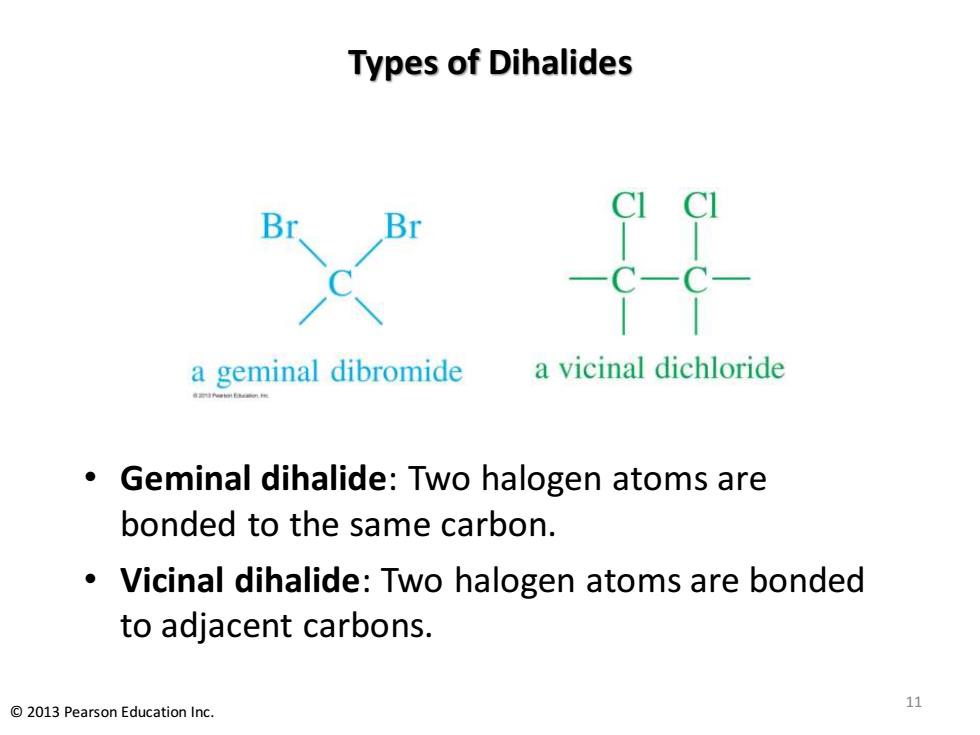
Types of Dihalides -C-C a geminal dibromide a vicinal dichloride Geminal dihalide:Two halogen atoms are bonded to the same carbon. Vicinal dihalide:Two halogen atoms are bonded to adjacent carbons. 11 2013 Pearson Education Inc
Types of Dihalides • Geminal dihalide: Two halogen atoms are bonded to the same carbon. • Vicinal dihalide: Two halogen atoms are bonded to adjacent carbons. 11 © 2013 Pearson Education Inc

Uses of Alkyl Halides Industrial and household cleaners. ·Anesthetics: CHCla used originally as general anesthetic but it is toxic and carcinogenic. CFaCHCIBr is a mixed halide sold as Halothane. Freons are used as refrigerants and foaming agents. Freons can harm the ozone layer,so they have been replaced by low-boiling hydrocarbons or carbon dioxide. Pesticides such as DDT are extremely toxic to insects but not as toxic to mammals. Haloalkanes can not be destroyed by bacteria,so they accumulate in the soil to a level that can be toxic to mammals,especially humans. 2013 Pearson Education Inc. 12
Uses of Alkyl Halides • Industrial and household cleaners. • Anesthetics: – CHCl3 used originally as general anesthetic but it is toxic and carcinogenic. – CF3CHClBr is a mixed halide sold as Halothane. • Freons are used as refrigerants and foaming agents. – Freons can harm the ozone layer, so they have been replaced by low-boiling hydrocarbons or carbon dioxide. • Pesticides such as DDT are extremely toxic to insects but not as toxic to mammals. – Haloalkanes can not be destroyed by bacteria, so they accumulate in the soil to a level that can be toxic to mammals, especially humans. 12 © 2013 Pearson Education Inc
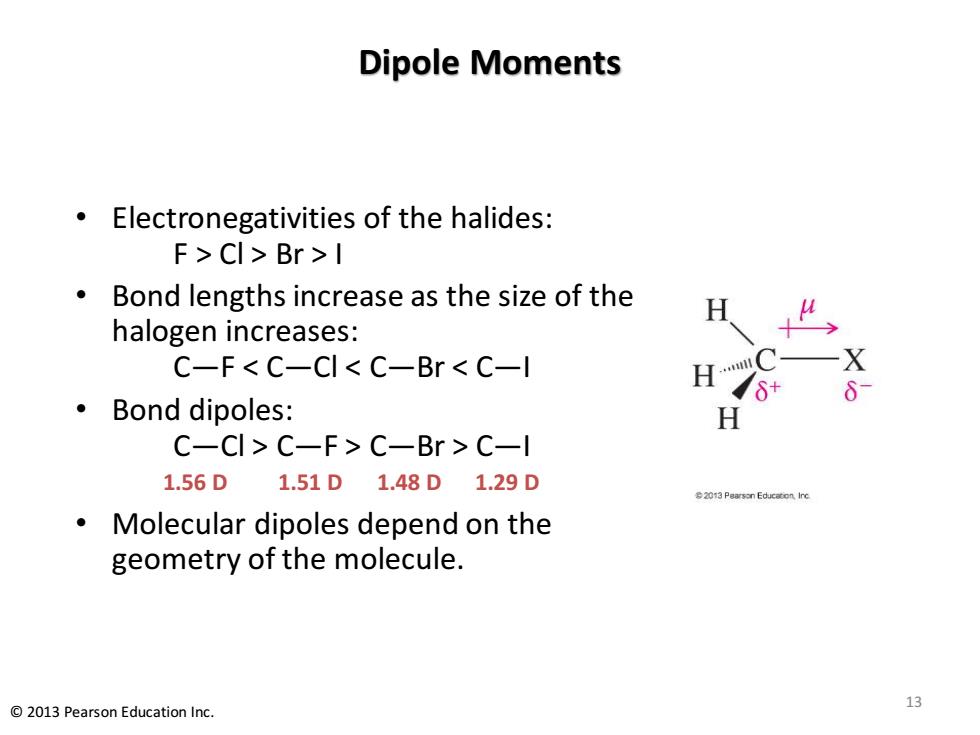
Dipole Moments Electronegativities of the halides: F>CI>Br>I Bond lengths increase as the size of the H halogen increases: C-F<C-CI<C-Br C-l X ·Bond dipoles: H C-CI>C-F>C-Br C-l 1.56D1.51D1.48D1.29D 2013 Pearson Educacon Ine Molecular dipoles depend on the geometry of the molecule. 13 2013 Pearson Education Inc
Dipole Moments • Electronegativities of the halides: F > Cl > Br > I • Bond lengths increase as the size of the halogen increases: C—F < C—Cl < C—Br < C—I • Bond dipoles: C—Cl > C—F > C—Br > C—I 1.56 D 1.51 D 1.48 D 1.29 D • Molecular dipoles depend on the geometry of the molecule. 13 © 2013 Pearson Education Inc
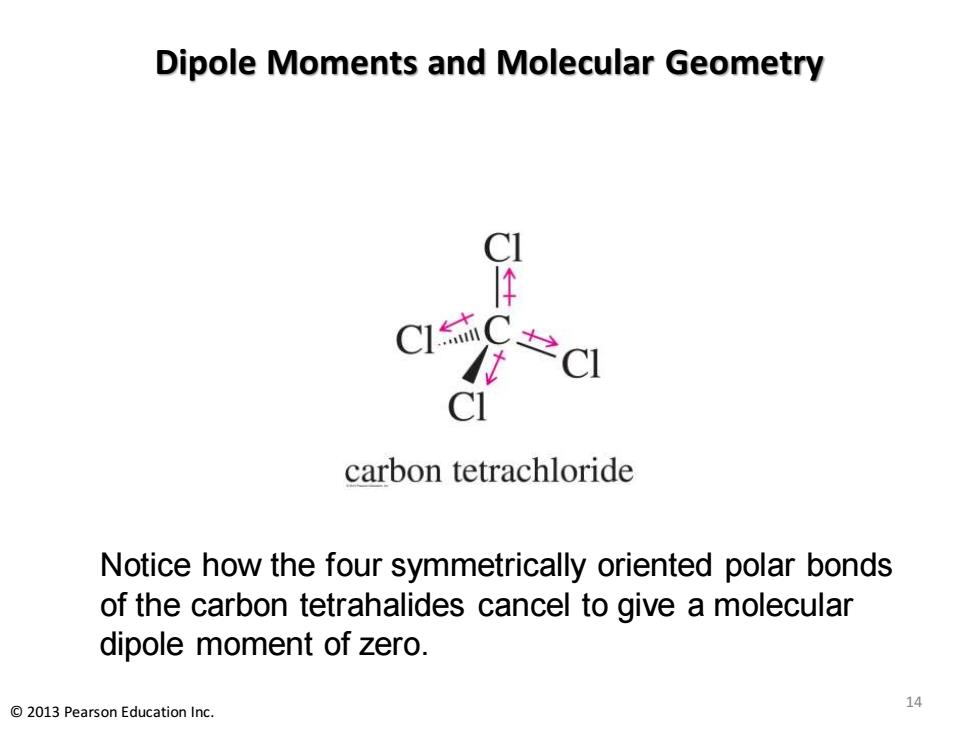
Dipole Moments and Molecular Geometry C C CI carbon tetrachloride Notice how the four symmetrically oriented polar bonds of the carbon tetrahalides cancel to give a molecular dipole moment of zero. 2013 Pearson Education Inc. 14
Dipole Moments and Molecular Geometry Notice how the four symmetrically oriented polar bonds of the carbon tetrahalides cancel to give a molecular dipole moment of zero. 14 © 2013 Pearson Education Inc
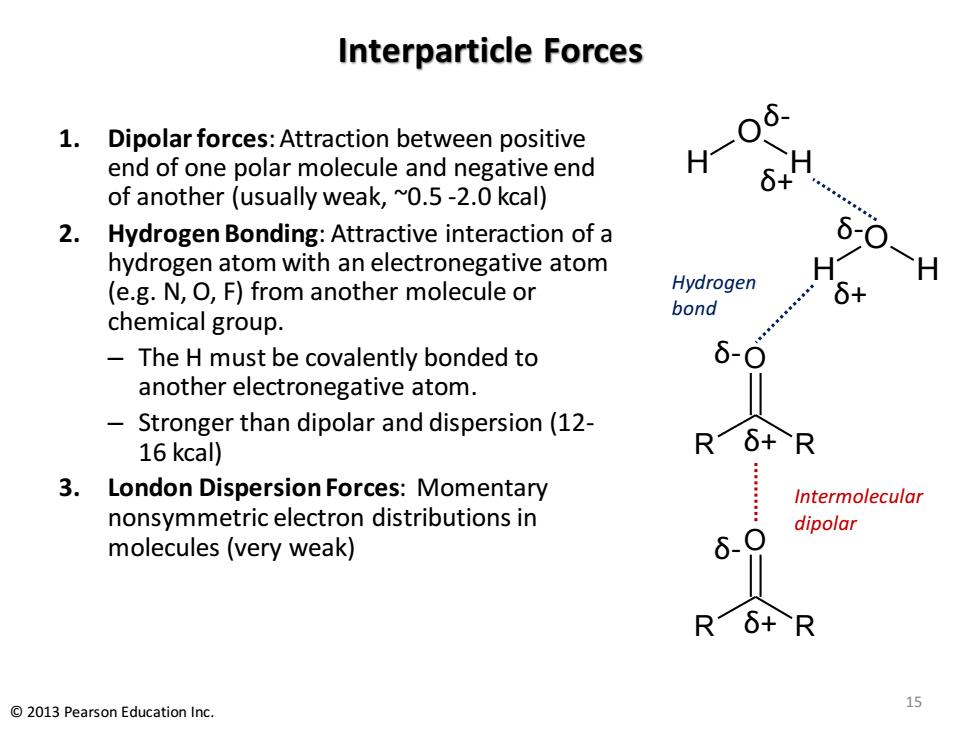
Interparticle Forces 6 1.Dipolar forces:Attraction between positive end of one polar molecule and negative end H- 6+ of another (usually weak,~0.5-2.0 kcal) 2. Hydrogen Bonding:Attractive interaction of a 6-0 hydrogen atom with an electronegative atom (e.g.N,O,F)from another molecule or Hydrogen 6+ bond chemical group. The H must be covalently bonded to 6-0 another electronegative atom. Stronger than dipolar and dispersion(12- 16 kcal) R6+R 3.London Dispersion Forces:Momentary Intermolecular nonsymmetric electron distributions in dipolar molecules (very weak) 6、O Rδ+R 2013 Pearson Education Inc. 15
Interparticle Forces 1. Dipolar forces: Attraction between positive end of one polar molecule and negative end of another (usually weak, ~0.5 -2.0 kcal) 2. Hydrogen Bonding: Attractive interaction of a hydrogen atom with an electronegative atom (e.g. N, O, F) from another molecule or chemical group. – The H must be covalently bonded to another electronegative atom. – Stronger than dipolar and dispersion (12- 16 kcal) 3. London Dispersion Forces: Momentary nonsymmetric electron distributions in molecules (very weak) O R R O R R δ- δ- δ+ δ+ Intermolecular dipolar H H O Hydrogen bond δ+ δ- H H O δ+ δ- 15 © 2013 Pearson Education Inc