
Chapter 1:Crystal Structure Objectives At the end of this Chapter,you should: 1.Be able to identify a unit cell in a symmetrical pattern. 2.Know that there are7 Possible unit cell shapes. 3.Be able to define cubic,tetragonal,orthorhombic hexagonal unit cell shapes. 4.Know how to index the crystal plane and the crystal direction. 5.Understand the difference between complex lattice and simple lattice 6.Understand the symmetry elements
Objectives At the end of this Chapter, you should: 1.Be able to identify a unit cell in a symmetrical pattern. 2. Know that there are7 Possible unit cell shapes. 3. Be able to define cubic, tetragonal, orthorhombic & hexagonal unit cell shapes. 4. Know how to index the crystal plane and the crystal direction. 5.Understand the difference between complex lattice and simple lattice 6. Understand the symmetry elements Chapter 1: Crystal Structure
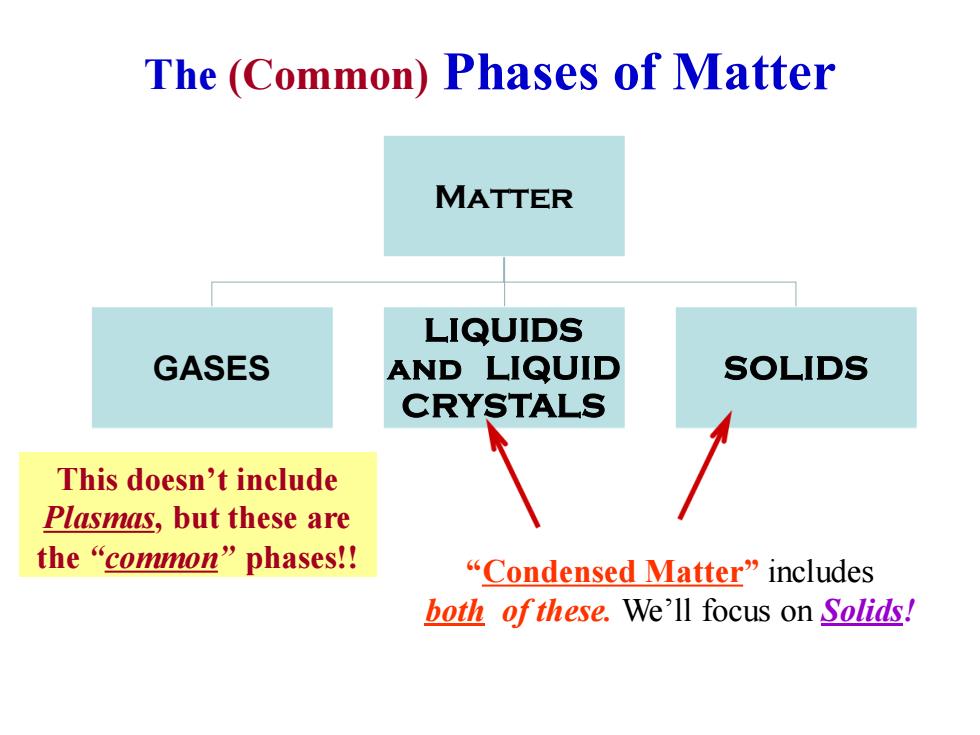
The (Common)Phases of Matter MATTER LIQUIDS GASES AND LIQUID SOLIDS CRYSTALS This doesn't include Plasmas,but these are the“common”phases! “Condensed Matter”includes both ofthese.We'll focus on Solids!
The (Common) Phases of Matter Matter GASES LIQUIDS and LIQUID CRYSTALS SOLIDS “Condensed Matter” includes both of these. We’ll focus on Solids! This doesn’t include Plasmas, but these are the “common” phases!!

1.1 Elementary Crystallography Solid Material Types Crystalline Polycrystalline Amorphous (Non-Crystalline) Single Crystal
1.1 Elementary Crystallography Solid Material Types Crystalline Polycrystalline Amorphous (Non-Crystalline) Single Crystal
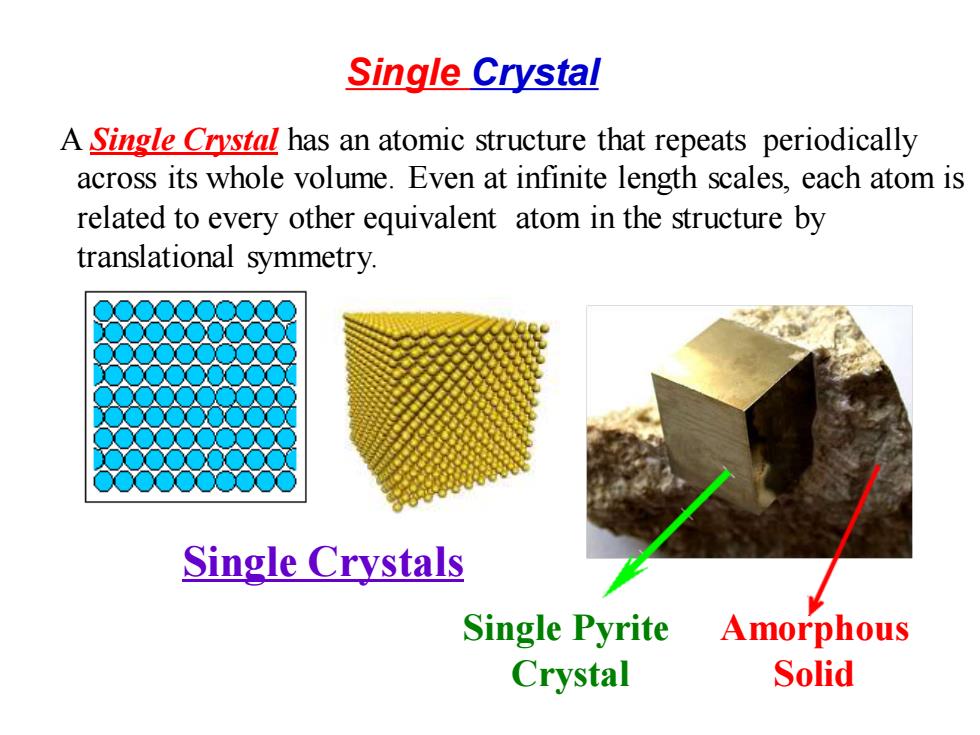
Single Crystal A Single Crystal has an atomic structure that repeats periodically across its whole volume.Even at infinite length scales,each atom is related to every other equivalent atom in the structure by translational symmetry. Single Crystals Single Pyrite Amorphous Crystal Solid
A Single Crystal has an atomic structure that repeats periodically across its whole volume. Even at infinite length scales, each atom is related to every other equivalent atom in the structure by translational symmetry. Single Crystals Single Pyrite Crystal Amorphous Solid Single Crystal
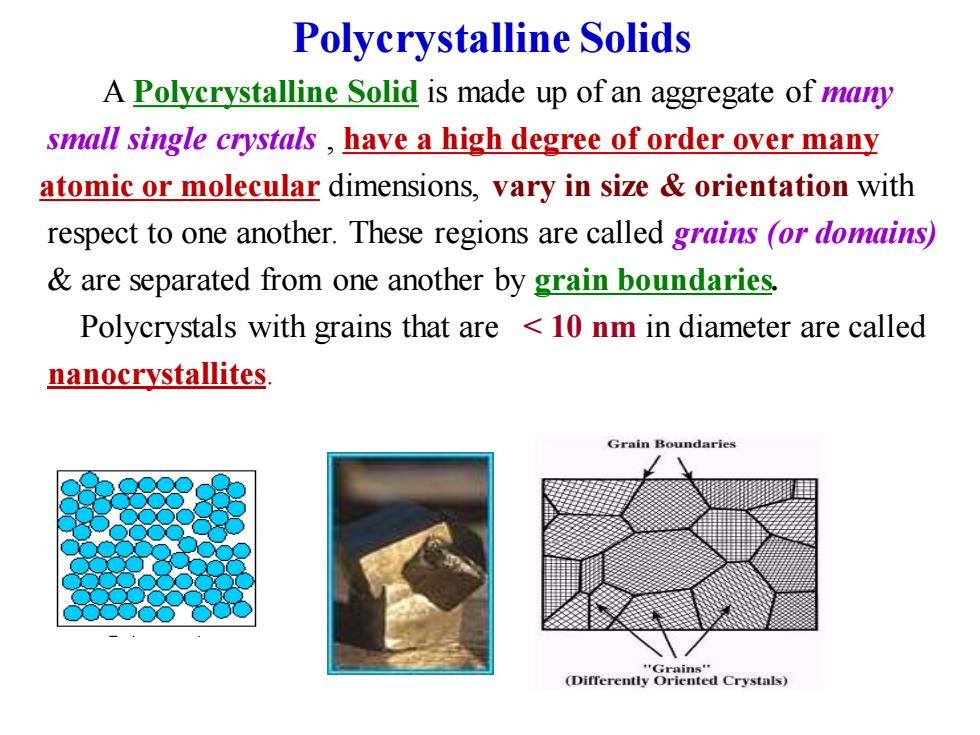
Polycrystalline Solids A Polycrystalline Solid is made up of an aggregate of many small single crystals have a high degree of order over many atomic or molecular dimensions,vary in size orientation with respect to one another.These regions are called grains (or domains) are separated from one another by grain boundaries. Polycrystals with grains that are 10 nm in diameter are called nanocrystallites Grain Boundaries (Differently red Crystals)
Polycrystalline Solids A Polycrystalline Solid is made up of an aggregate of many small single crystals , have a high degree of order over many atomic or molecular dimensions, vary in size & orientation with respect to one another. These regions are called grains (or domains) & are separated from one another by grain boundaries. Polycrystals with grains that are < 10 nm in diameter are called nanocrystallites