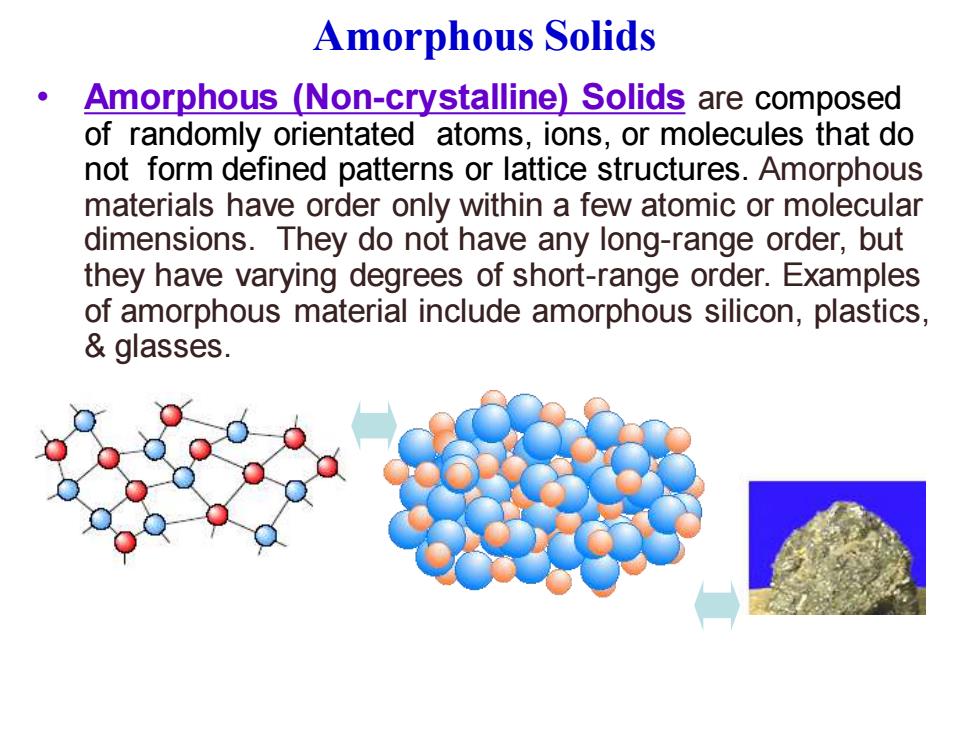
Amorphous Solids Amorphous (Non-crystalline)Solids are composed of randomly orientated atoms,ions,or molecules that do not form defined patterns or lattice structures.Amorphous materials have order only within a few atomic or molecular dimensions.They do not have any long-range order,but they have varying degrees of short-range order.Examples of amorphous material include amorphous silicon,plastics, glasses
Amorphous Solids • Amorphous (Non-crystalline) Solids are composed of randomly orientated atoms, ions, or molecules that do not form defined patterns or lattice structures. Amorphous materials have order only within a few atomic or molecular dimensions. They do not have any long-range order, but they have varying degrees of short-range order. Examples of amorphous material include amorphous silicon, plastics, & glasses

1.2 Crystal Structure Lattice Basis Lattice:arranging way Basis:group of atoms arranged Crystal ● ● ● ● ,● ● ●】 ●● ● ●● ●● Basis Lattice=Crystal
• Lattice: arranging way • Basis: group of atoms arranged 1.2 Crystal Structure ≡ Lattice + Basis
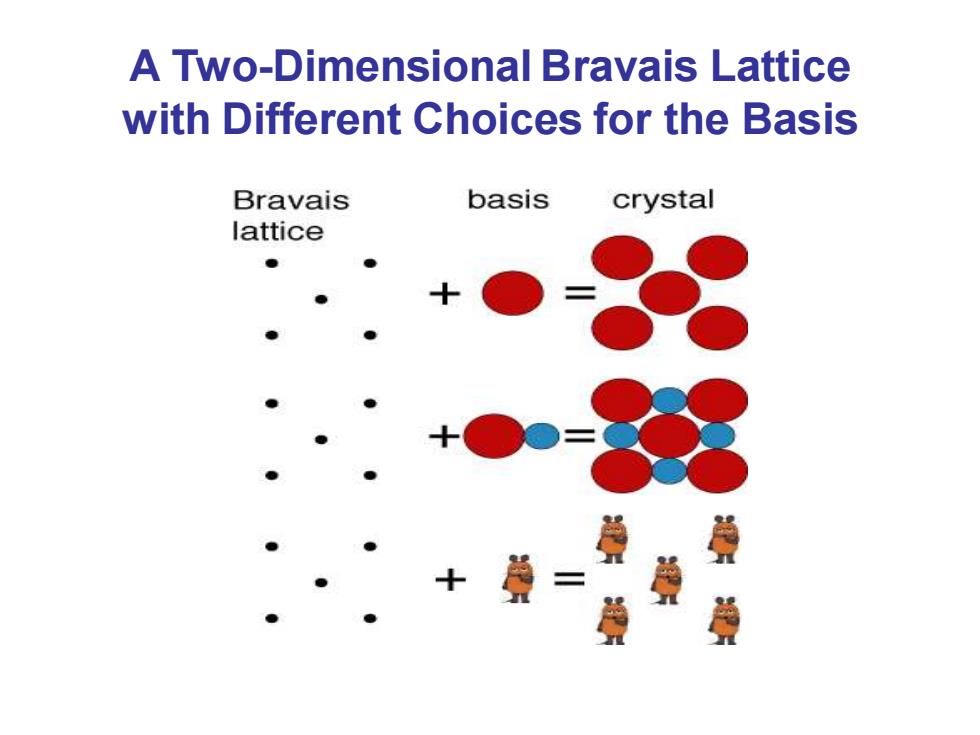
A Two-Dimensional Bravais Lattice with Different Choices for the Basis Bravais basis crystal lattice ●
A Two-Dimensional Bravais Lattice with Different Choices for the Basis