
3.Interatomic Bonding Objectives At the end of this Chapter,you should: 1.Be able to identify the bonding type in the solids. 2.Be able to calculate the cohesive energy and the related physics parameters for ionic crystals and covalent crystals 3.Understand the concept of Madelung constant
3. Interatomic Bonding Objectives At the end of this Chapter, you should: 1.Be able to identify the bonding type in the solids. 2. Be able to calculate the cohesive energy and the related physics parameters for ionic crystals and covalent crystals 3. Understand the concept of Madelung constant
3.1 General crystal binding The stable bonding arrangement:the spatial configuration of positive ion cores outer electrons has a smaller QUANTUM MECHANICAL TOTAL ENERGY than any other configuration of these particles The Cohesive Energy:the energy difference of the configuration of atoms compared with that of the isolated atoms.~0.1 eV/atom for solids ~7 eV/atom or greater in some covalent some ionic compounds some metals. Each bonding mechanism between the atoms in a solid is a result of the electrostatic interaction between the nuclei the electrons
3.1 General crystal binding The stable bonding arrangement: the spatial configuration of positive ion cores & outer electrons has a smaller QUANTUM MECHANICAL TOTAL ENERGY than any other configuration of these particles The Cohesive Energy: the energy difference of the configuration of atoms compared with that of the isolated atoms. ~ 0.1 eV/atom for solids ~ 7 eV/atom or greater in some covalent & some ionic compounds & some metals. Each bonding mechanism between the atoms in a solid is a result of the electrostatic interaction between the nuclei & the electrons
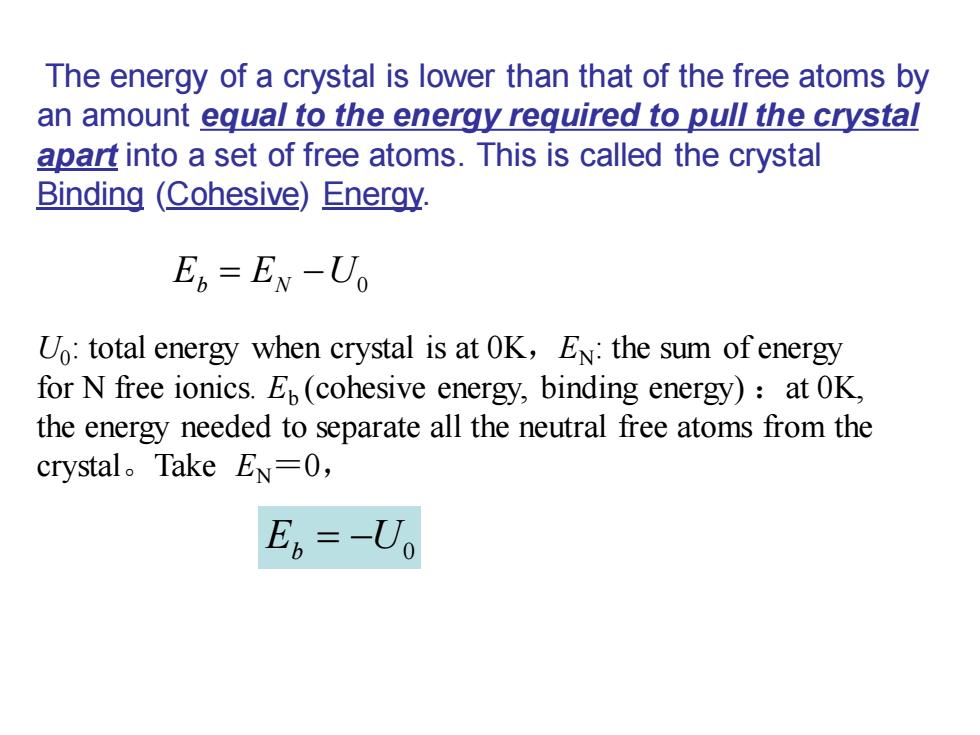
The energy of a crystal is lower than that of the free atoms by an amount equal to the energy required to pull the crystal apart into a set of free atoms.This is called the crystal Binding (Cohesive)Enerqy. En=EN-U Uo:total energy when crystal is at OK,EN:the sum of energy for N free ionics.E(cohesive energy,binding energy):at OK, the energy needed to separate all the neutral free atoms from the crystal。Take EN=O, E6=-U
The energy of a crystal is lower than that of the free atoms by an amount equal to the energy required to pull the crystal apart into a set of free atoms. This is called the crystal Binding (Cohesive) Energy. E E U b N = − 0 E U b = − 0 U0 : total energy when crystal is at 0K,EN: the sum of energy for N free ionics. Eb (cohesive energy, binding energy) :at 0K, the energy needed to separate all the neutral free atoms from the crystal。Take EN =0
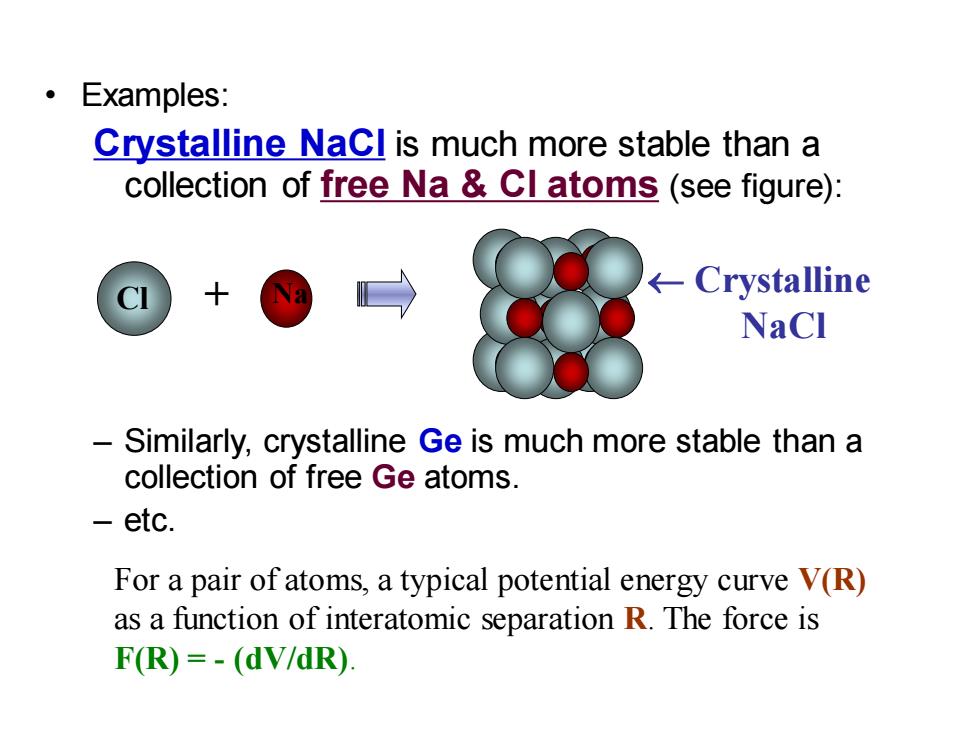
Examples: Crystalline Nacl is much more stable than a collection of free Na Cl atoms (see figure): Crystalline NaCl Similarly,crystalline Ge is much more stable than a collection of free Ge atoms. -etc. For a pair of atoms,a typical potential energy curve V(R) as a function of interatomic separation R.The force is F(R)=-(dV/dR)
• Examples: Crystalline NaCl is much more stable than a collection of free Na & Cl atoms (see figure): – Similarly, crystalline Ge is much more stable than a collection of free Ge atoms. – etc. Na Crystalline NaCl Cl + For a pair of atoms, a typical potential energy curve V(R) as a function of interatomic separation R. The force is F(R) = - (dV/dR)
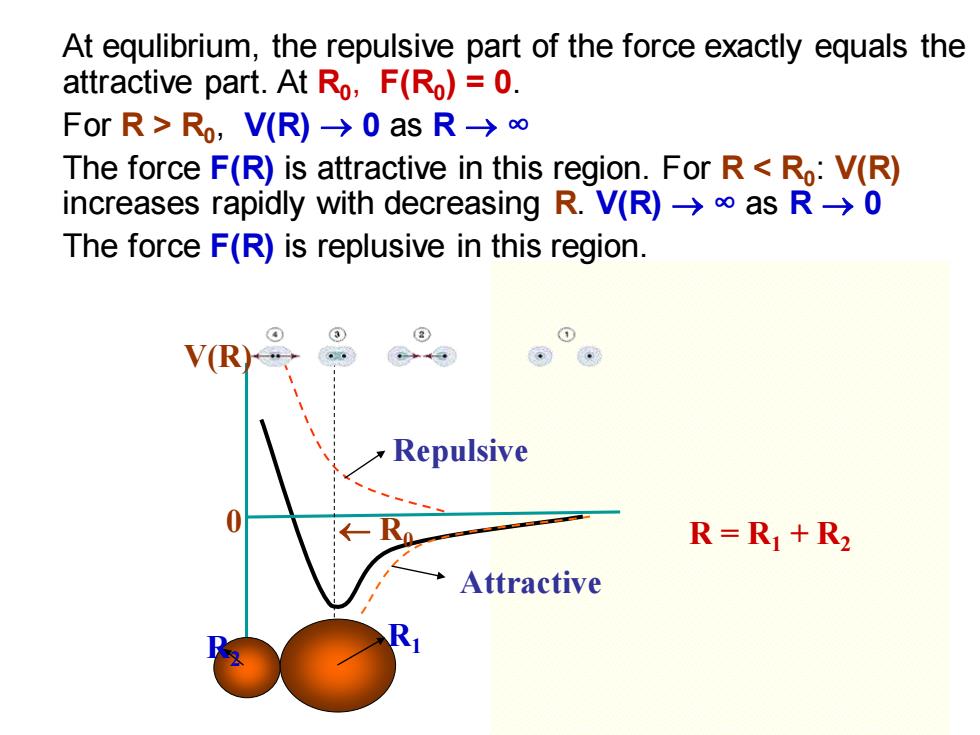
At equlibrium,the repulsive part of the force exactly equals the attractive part.At Ro,F(Ro)=0. ForR>Ro,V(R)-0asR→∞ The force F(R)is attractive in this region.For R<Ro:V(R) increases rapidly with decreasing R.V(R)>as R->0 The force F(R)is replusive in this region. V(R Repulsive 0 R=R1+R2 Attractive
R2 R1 V(R) 0 R0 Repulsive Attractive At equlibrium, the repulsive part of the force exactly equals the attractive part. At R0 , F(R0 ) = 0. For R > R0 , V(R) → 0 as R → ∞ The force F(R) is attractive in this region. For R < R0 : V(R) increases rapidly with decreasing R. V(R) → ∞ as R → 0 The force F(R) is replusive in this region. R = R1 + R2