
3-1 supplement:Rules of crystal binding Electronegative:used to characterize the ability of losing or getting electrons.In the periodical tables,the Electronegativity is increased from the left to right in the same period,and is reduced from the top to down Mulliken definition: Electronegativity=0.18 (ionization energy+electron affinity)
3-1 supplement:Rules of crystal binding Electronegative:used to characterize the ability of losing or getting electrons. In the periodical tables, the Electronegativity is increased from the left to right in the same period, and is reduced from the top to down Mulliken definition: Electronegativity=0.18(ionization energy+electron affinity)
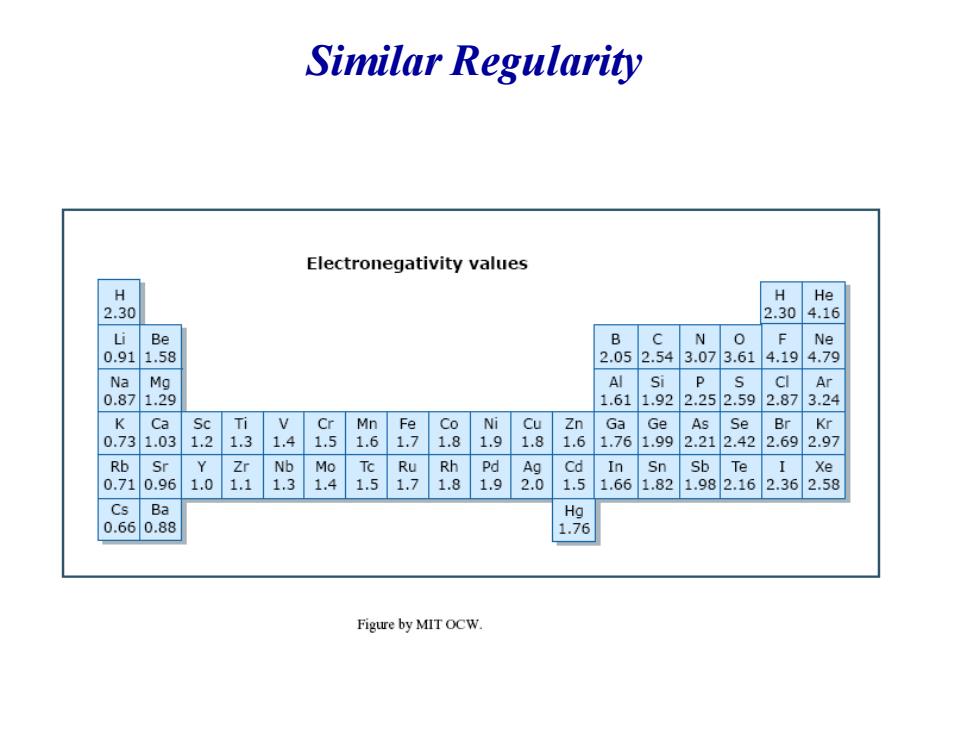
Similar Regularity Electronegativity values H H He 2.30 2.304.16 Be B C N 0 F Ne 0.91 1.58 2.052.543.073.61 4.194.79 Na Si P Ar 0.87 1.611.922.252.592.873.24 K Ca Sc Ti Mn Fe Co Ni Cu Ga Ge As Se Br Kr 0.73 1.03 1.2 1.3 1.4 .5 1.6 1.8 1.9 1.8 1.6 1.76 1.99 2.212.42 2.692.97 Rb Sr Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe 0.71 0.96 1.0 1.1 1.3 1.4 1.5 1.7 1.8 1.9 2.0 1.5 1.66 1.82 1.982.162.362.58 Cs Ba Ha 0.660.88 1.76 Figure by MIT OCW
Similar Regularity
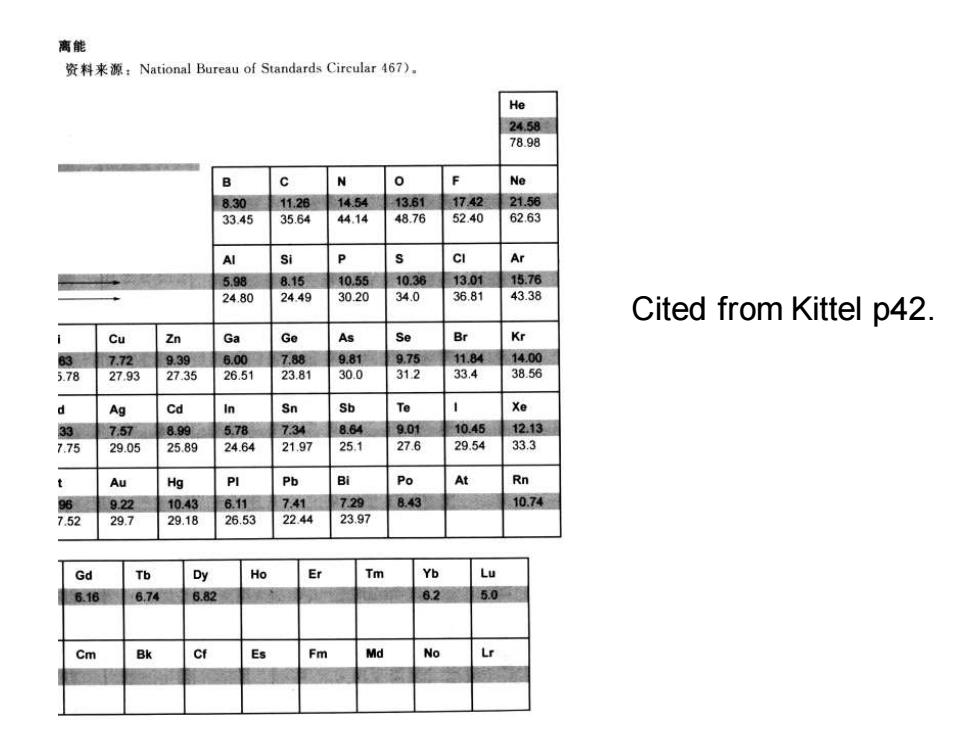
离能 资料来源:National Bureau of Standards Circular467). He 24.58 78.98 B c N 0 Ne 8.30 11.26 14.54 13.61 1742 21.56 33.45 35.64 44.14 48.76 52.40 62.63 Si CI Ar 5.98 8.15 10.55 10.38 1301 15.76 24.80 24.49 30.20 34.0 36.81 43.38 Cited from Kittel p42 Cu Zn Ga Ge AS Se Br Kr 3 7.72 9.39 6.00 7,88 9.81 9.75 11.84 14.00 5.78 27.93 27.35 26.51 23.81 30.0 312 33.4 38.56 Ag Cd In Sn Sb Xe 33 7.57 899 578 7.34 8.64 9.01 10.45 12.13 775 29.05 25.89 24.64 21.97 25.1 27.6 29.54 33.3 Au Hg Pb Po At Rn 96 922 10.43 6.11 7.41 729 8.43 10.74 7.52 29.7 29.18 26.53 22.44 23.97 Gd Tb Dy Ho Er Tm Yb 6.16 6.74 6.82 82 5.0 Cm Bk Fm Md No
Cited from Kittel p42

General rules for crystal binding lowest electronegativity for alkali metal,liable to lose elctrons *IV-VI elements possess a higher electronegativity,and strong ability to obtain electrons,liable to form covalent bonds.IVelements are typical example.C (diamond)have biggest electronegativity, and have a strong covalent bond,while Pb is weakly electronegative and is a mental The value of electronegativity for elements at both end of the periodical tables have a big differnce,so,I -VII elements are lible to form ionic crystal,for example,NaCl,CsCl. With the reduction of the electronegativity difference,the ionic binding gradually is tranformed to covalent bingding
★ The value of electronegativity for elements at both end of the periodical tables have a big differnce, so , Ⅰ-Ⅶ elements are lible to form ionic crystal , for example, NaCl,CsCl. ★ With the reduction of the electronegativity difference,the ionic binding gradually is tranformed to covalent bingding. 。 General rules for crystal binding ★ lowest electronegativity for alkali metal, liable to lose elctrons ★ Ⅳ-Ⅵ elements possess a higher electronegativity, and strong ability to obtain electrons, liable to form covalent bonds. Ⅳelements are typical example. C(diamond) have biggest electronegativity, and have a strong covalent bond, while Pb is weakly electronegative and is a mental
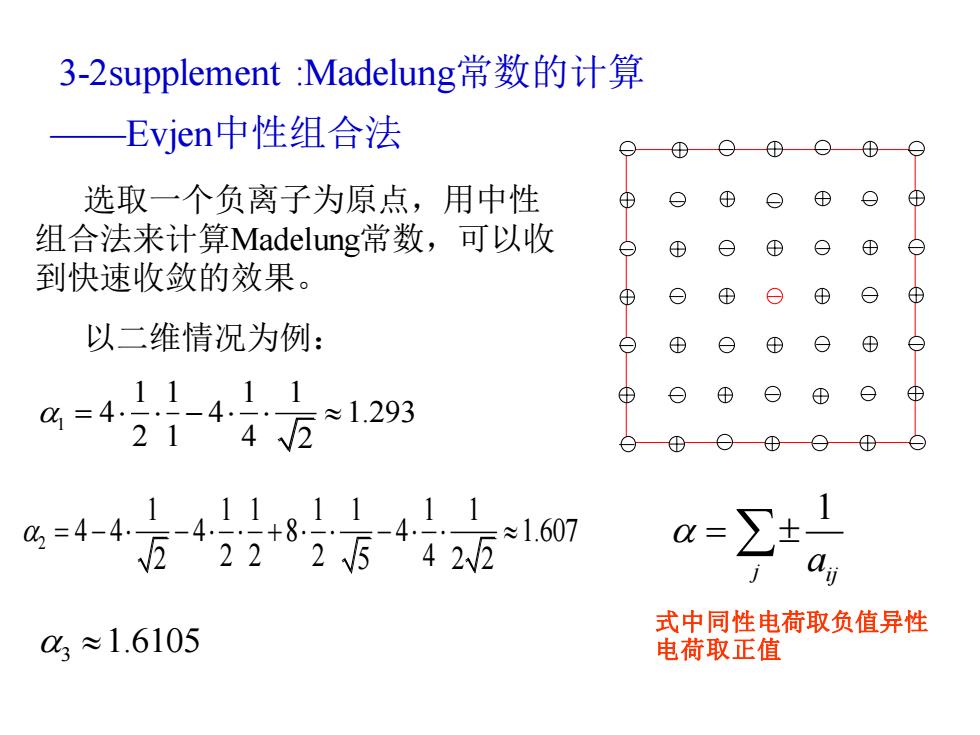
3-2 supplement:Madelung常数的计算 Evjen中性组合法 ⊕ O⊕ 选取一个负离子为原点,用中性 ⊕ ⊕ o ⊕ ⊕ 组合法来计算Madelung常数,可以收 ⊕ ⊕ ⑧ 到快速收敛的效果。 ⊕ ⊕ ⊕ 以二维情况为例: ⊕ 4=4)4161293 ⊕ 214√2 2=446-4+851-411 -4-+8 迈42225中422 ≈1.607 03≈1.6105 式中同性电荷取负值异性 电荷取正值
3-2supplement :Madelung常数的计算 ——Evjen中性组合法 选取一个负离子为原点,用中性 组合法来计算Madelung常数,可以收 到快速收敛的效果。 以二维情况为例: 1 1 1 1 1 4 4 1.293 2 1 4 2 = − 2 1 1 1 1 1 1 1 4 4 4 8 4 1.607 2 5 2 2 2 2 2 4 = − − + − 3 1.6105 1 j ij a = 式中同性电荷取负值异性 电荷取正值