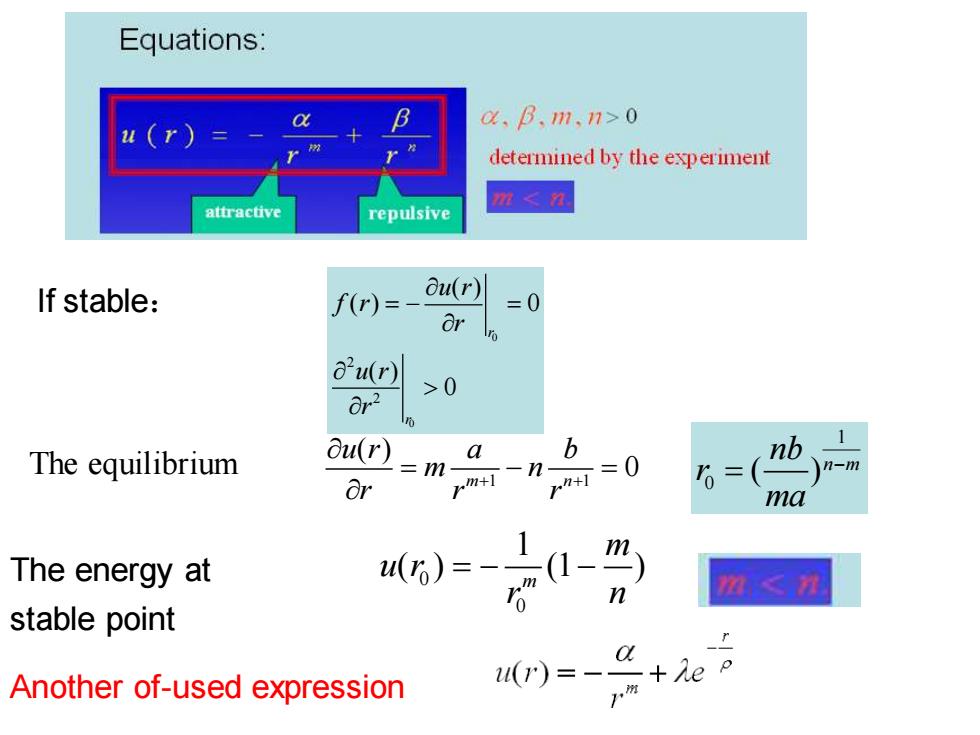
Equations: u(r)= a,B.m.n>o determined by the experiment attractive repulsive 鞋≤ If stable: f(r)=- ou(r) =0 u(r) 8r2 >0 The equilibrium ou(r)=m- a b *0 nb m+1 -n- n-m Or 6=( ma The energy at ()=- (1-) m,≤习 stable point Another of-used expression (r)=- +Ae
If stable: 0 0 2 2 ( ) ( ) 0 ( ) 0 r r u r f r r u r r = − = The equilibrium 0 0 1 ( ) (1 ) m m u r r n The energy at = − − stable point 1 1 ( ) 0 m n u r a b m n r r r + + = − = 1 0 ( )n m nb r ma − = Another of-used expression
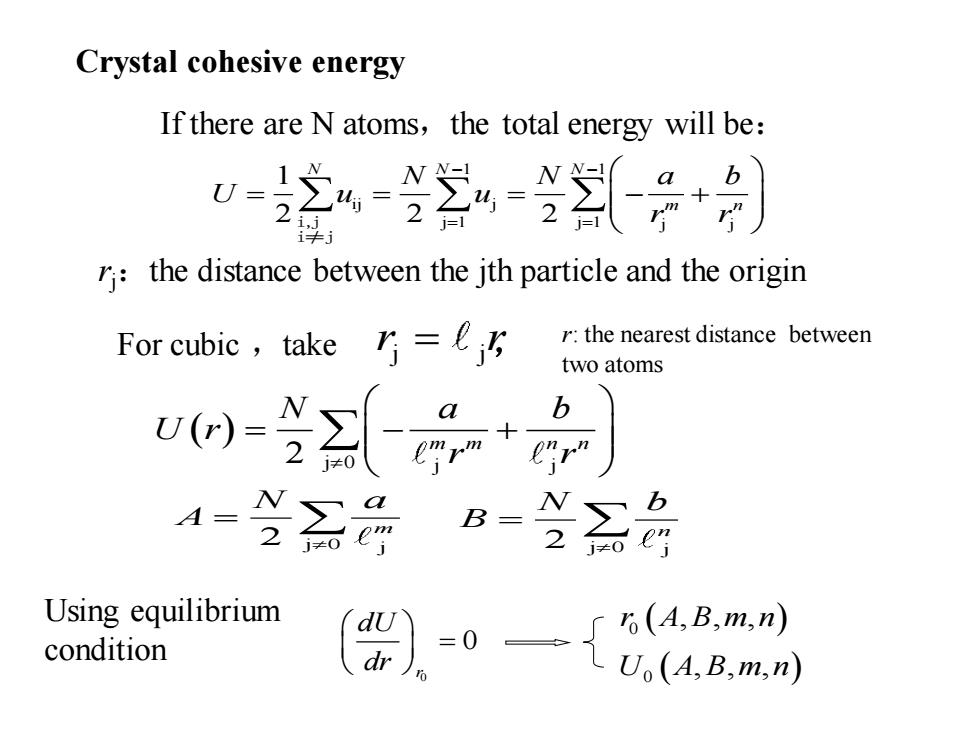
Crystal cohesive energy If there are N atoms,the total energy will be: w之4器=÷】 2 r:the distance between the jth particle and the origin For cubic,take r:the nearest distance between two atoms b A- B= 2o Using equilibrium 6(A,B,m,n) condition
If there are N atoms,the total energy will be: 1 1 ij j j 1 j 1 j j 1 2 2 2 N N N m n N N a b U u u r r − − = = = = = − + i,j i≠j ( ) 2 j 0 j j m m n n N a b U r r r = − + j j For cubic ,take r r = , Crystal cohesive energy rj:the distance between the jth particle and the origin r: the nearest distance between two atoms 2 j 0 j m N a A = 2 j 0 j n N b B = 0 0 r dU dr = Using equilibrium condition ( ) ( ) 0 0 , , , , , , r A B m n U A B m n
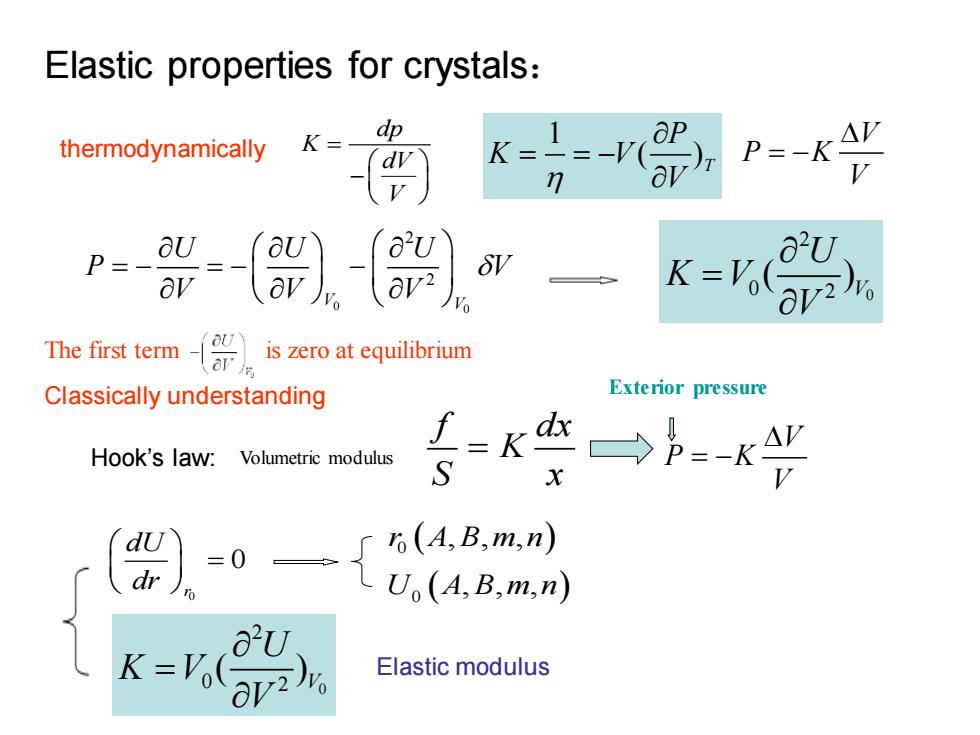
Elastic properties for crystals: dp thermodynamically K= k(ar) P=-KA Ps aU a"U av K-Voav2 The first term is zero at equilibrium Classically understanding Exterior pressure Hook's law:Volumetric modulus S 6(A,B,m,n) U (A,B,m,n) K=Vo( a U Elastic modulus
Elastic properties for crystals: 1 ( )T P K V V = = − thermodynamically V P K V = − 0 0 2 2 V V U U U P V V V V = − = − − 0 2 0 2 ( )V U K V V = dp K dV V = − The first term is zero at equilibrium Classically understanding Hook’s law: f dx K S x = V P K V = − Exterior pressure Volumetric modulus 0 0 r dU dr = ( ) ( ) 0 0 , , , , , , r A B m n U A B m n 0 2 0 2 ( )V U K V V = Elastic modulus
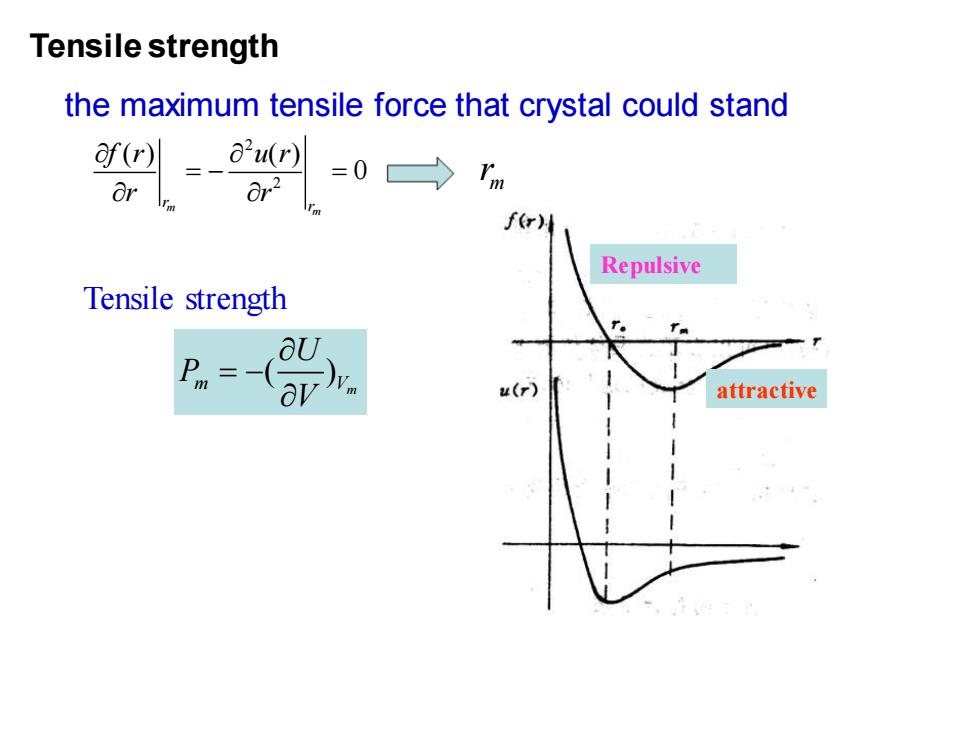
Tensile strength the maximum tensile force that crystal could stand f四=-aw =01m f(r) Repulsive Tensile strength aU Pm= av attractive
Tensile strength the maximum tensile force that crystal could stand ( ) m Vm U P V = − m r Repulsive attractive 2 2 ( ) ( ) 0 m m r r f r u r r r = − = m r Tensile strength
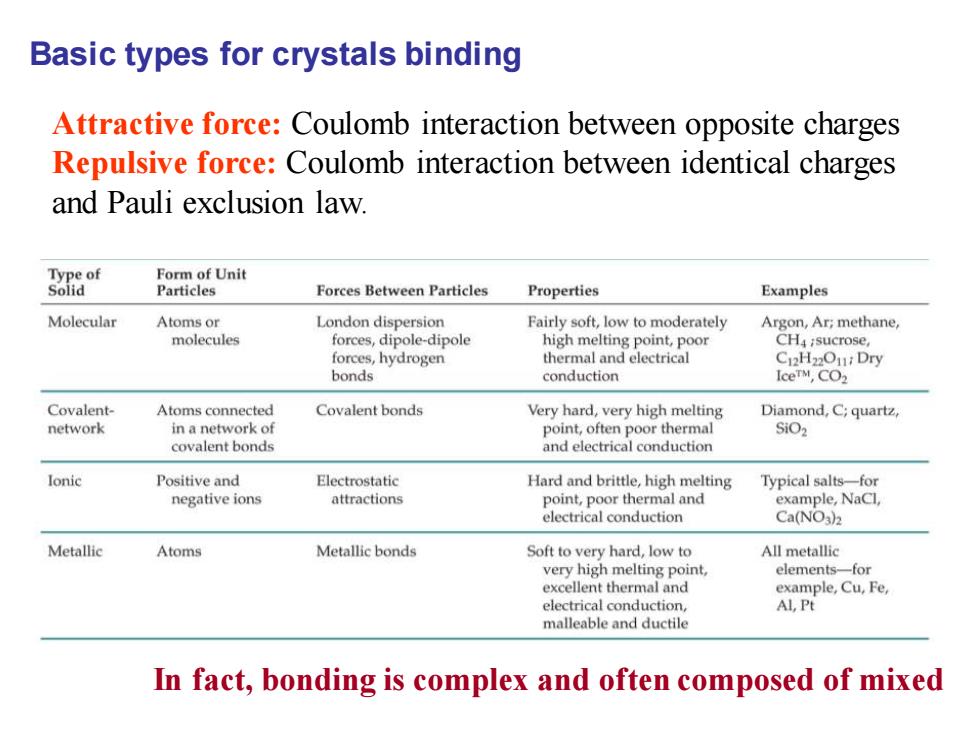
Basic types for crystals binding Attractive force:Coulomb interaction between opposite charges Repulsive force:Coulomb interaction between identical charges and Pauli exclusion law. Type of Form of Unit Solid Particles Forces Between Particles Properties Examples Molecular Atoms or London dispersion Fairly soft,low to moderately Argon,Ar;methane, molecules forces,dipole-dipole high melting point,poor CH4;sucrose, forces,hydrogen thermal and electrical C2H2O1:Dry bonds conduction IceTM,CO2 Covalent- Atoms connected Covalent bonds Very hard,very high melting Diamond,C;quartz, network in a network of point,often poor thermal SiO2 covalent bonds and electrical conduction Ionic Positive and Electrostatic Hard and brittle,high melting Typical salts-for negative ions attractions point,poor thermal and example,NaCl. electrical conduction Ca(NO3)2 Metallic Atoms Metallic bonds Soft to very hard,low to All metallic very high melting point, elements-for excellent thermal and example,Cu,Fe, electrical conduction, Al,Pt malleable and ductile In fact,bonding is complex and often composed of mixed
Basic types for crystals binding Attractive force: Coulomb interaction between opposite charges Repulsive force: Coulomb interaction between identical charges and Pauli exclusion law. In fact, bonding is complex and often composed of mixed