
3.2 typical binding Ionic bingding:(alkali Li,Na,K,Rb,Cs chloride F,Cl,Br,D) NaCl:(coordination number,6) CsCl (coordination number,8) semiconductor-Cds ZnS. transfer of electrons between two atoms with a large difference in electro-negativities. ionics worked as the combining unit,and electrons are highly located around the ion core,forming the stable electron shell with spherical symmetry,no direction and saturation.So,closed packing principle could be applied for ionics,which could be regarded as a grid sphere. Rb+ CI- Cs→Xa
3.2 typical binding Ionic bingding: (alkali :Li,Na,K,Rb,Cs ; chloride : F,Cl,Br,I) transfer of electrons between two atoms with a large difference in electro-negativities. ionics worked as the combining unit,and electrons are highly located around the ion core, forming the stable electron shell with spherical symmetry, no direction and saturation. So, closed packing principle could be applied for ionics, which could be regarded as a grid sphere. NaCl:(coordination number,6) CsCl :(coordination number, 8) semiconductor —— CdS、ZnS
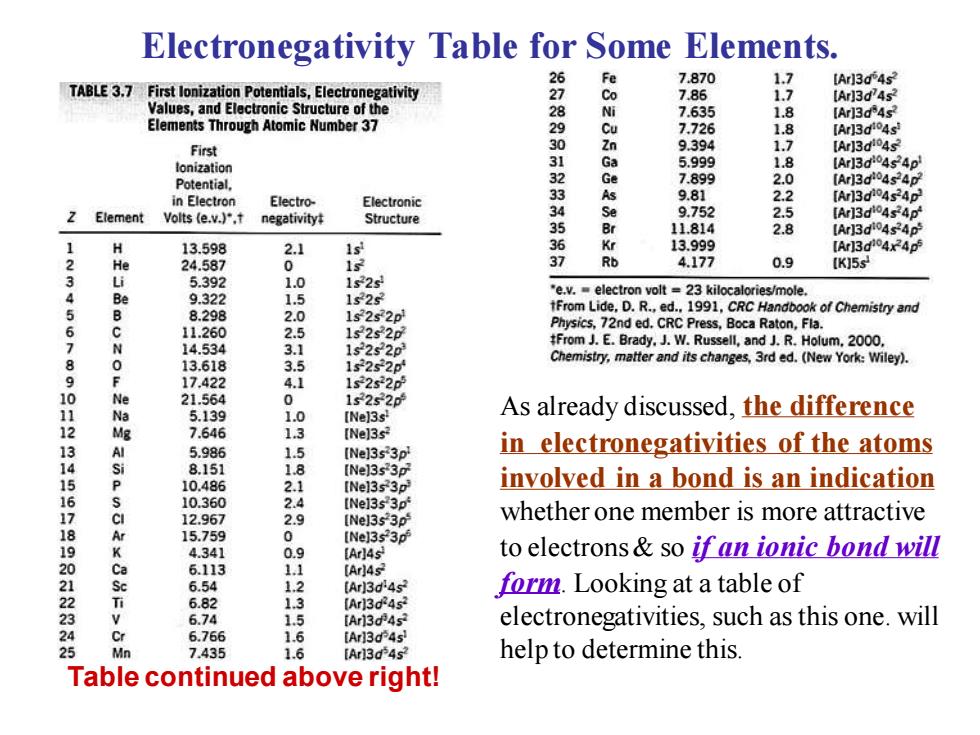
Electronegativity Table for Some Elements. Fe 7870 1.7 [Arl3d 4s TABLE 3.7 First lonization Potentials,Electronegativity 27 Co 7.86 1.7 [Arl3d4s Values,and Electronic Structure of the 7.635 1.8 [Ar13d4s? Elements Through Atomic Number 37 7.726 1.8 [Arl3d4s First 30 9.394 1.7 【Arj3dio4s lonization Potential. Ga 5.999 1.8 [Ar13d4s4p 7.899 2.0 [Arl3d04s4D in Electron Electro- Electronic 33 As 9.81 2.2 Z Element Volts (e.v.),t negativity Structure 3 9.752 2.5 [Ar13d04s4p 35 11,814 2.8 [Ar]3d04s40 13.598 .1 ls 新 13.999 [Ar13d04x4 2 He 24.587 0 Rb 4.177 0.9 [K]5s 3 5.392 1.0 1s2 9.322 1.5 1s25 "e.v.electron volt 23 kilocalories/mole. 567 8.298 2.0 1s252p tFrom Lide,D.R.,ed.1991,CRC Handbook of Chemistry and 11.260 Physics,72nd ed.CRC Press,Boca Raton,Fla. 2.5 1s2s2p 14.534 3.1 1s2s2p #From J.E.Brady.J.W.Russell,and J.R.Holum,2000, 89 13.618 3.5 1s2s2p Chemistry,matter and its changes,3rd ed.(New York:Wiley) 17.422 1s2s2p e 21.564 1s2s2p 5.139 1.0 [Ne]3s! As already discussed,the difference 12 Mg 7.646 1.3 [Ne]3s 13 Ev 5.986 4 8.151 [Ne]3s3p in electronegativities of the atoms [Ne]3s3p 15 10.486 21 [Ne]3s3p involved in a bond is an indication 16 10.360 2.4 [Nel3s3p 17 12.967 [Ne]3s3p whether one member is more attractive 15.759 [Ne]3s3p 4.341 0.9 (Arl4s to electrons so if an ionic bond will 20 6113 11 [Ar)4s 4223 6.54 (Ar)3d'4s form.Looking at a table of 6.82 [Ar3d453 6.74 1.5 [Ar13d4s electronegativities,such as this one.will 6.766 1.6 [Ar13d4s 7.435 1.6 [Ar13d4s? help to determine this. Table continued above right!
As already discussed, the difference in electronegativities of the atoms involved in a bond is an indication whether one member is more attractive to electrons & so if an ionic bond will form. Looking at a table of electronegativities, such as this one. will help to determine this. Electronegativity Table for Some Elements. Table continued above right!