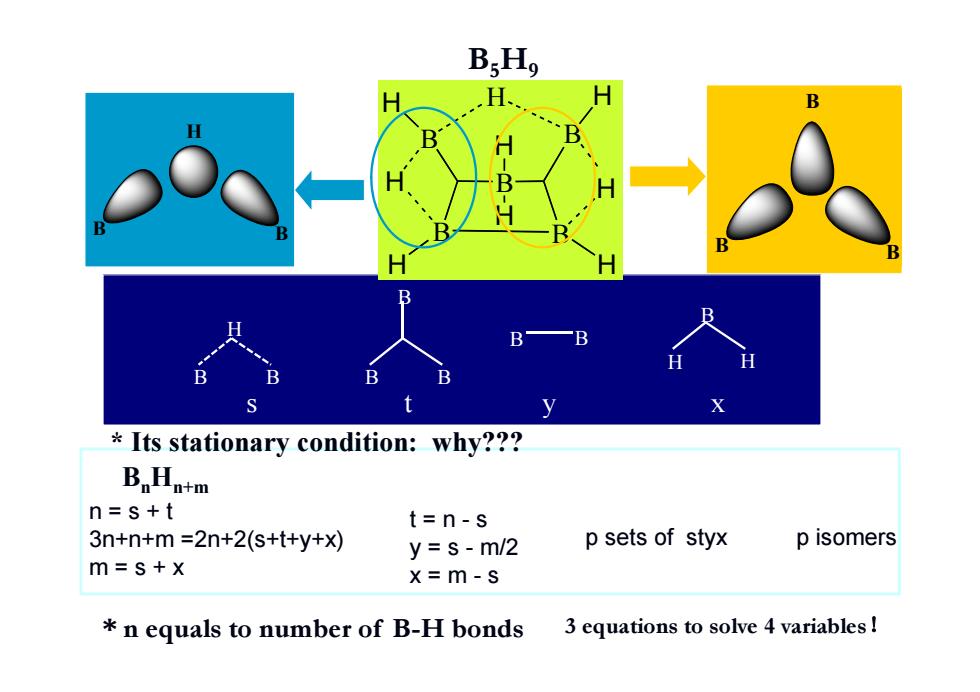
B;Ho H、 B B B y Its stationary condition:why??? BaHntm n=s+t t=n-s 3n+n+m=2n+2(s+t+y+x) y=s-m/2 p sets of styx p isomers m=s+x x=m-s *n equals to number of B-H bonds 3 equations to solve 4 variables
B H H B H H B H B H B H H H B B H B B B t=n-s y = s - m/2 x = m - s p sets of styx p isomers BnHn+m B5H9 * n equals to number of B-H bonds * Its stationary condition: why??? n=s+t 3n+n+m =2n+2(s+t+y+x) m = s + x B B H B B B B H H B B s ty x 3 equations to solve 4 variables!

n=6,m=4 styx ThetopologicalstnuctureofBgH,oy=gnw2-g-2 (2402) → (3 isomers) x=m-s=4-s (3311) (s=2,3,4) (4220) H H H BH2 BH2 H H H H H (4220) (3311) (2402) B y
B H B H H H B B B B H H H H H H B B B B B B H H H H H H H H H H H H H B B BH2 BH2 B B H (4220) (3311) (2402) H The topological structure of B6H10 (3 isomers) H Example 1: t=n-s=6-s y = s - m/2 = s - 2 x = m - s = 4 – s (s = 2, 3, 4) B B H B B B B H H B B s ty x n=6, m=4 styx (2402) (3311) (4220)

Example 3: The topological structure of B4Hi0 (3 isomers) t=n-s=4-s styx n=4,m=6 y=s-m/2=s-3 x=m-s=6-s (3103) (s=3,4) (4012) Expt.Structure H、 B (4012) (3103) Structure (4012)agrees well with the experimental data
The topological structure of B 4 H10 (3 isomers) Example 3: t = n - s = 4- s y = s - m/2 = s - 3 x = m - s = 6 – s (s = 3, 4) styx (3103) (4012) n=4, m=6 Structure (4012) agrees well with the experimental data
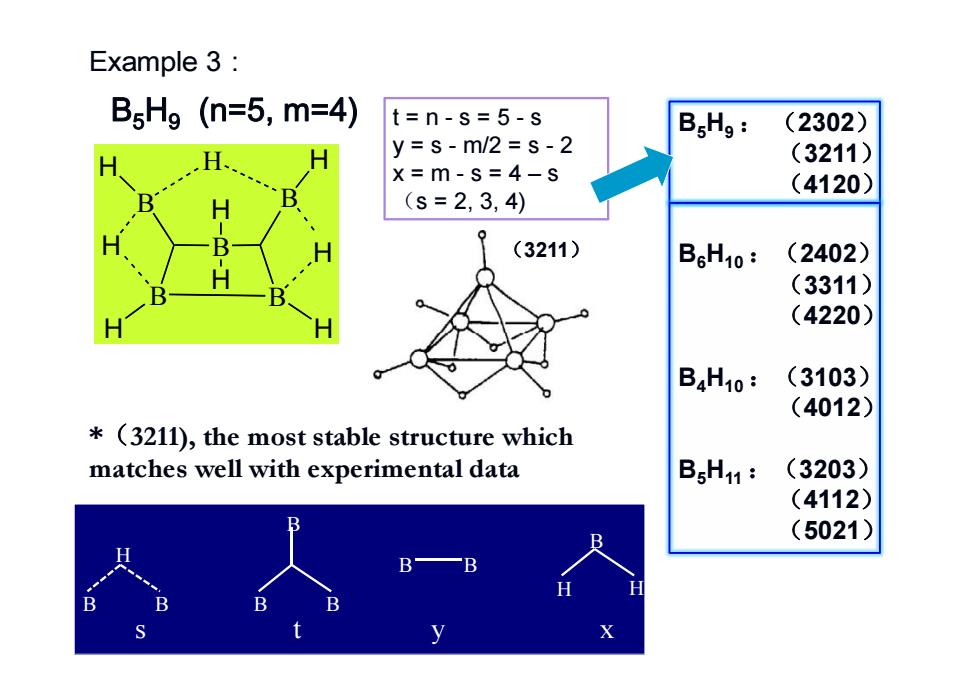
Example 3: B Hg (n=5,m=4) t=n-s=5-s BgHg: (2302) y=s-m/2=s-2 (3211) x=m-s=4-s (4120) (s=2,3,4) (3211) B6H10: (2402) (3311) (4220) B4H10: (3103) (4012) *(3211),the most stable structure which matches well with experimental data BgH11: (3203) (4112) B (5021) B B B B X
B H H B H H B H B H B H H H B5H9 (n=5, m=4) t=n-s=5-s y = s - m/2 = s - 2 x = m - s = 4 – s (s = 2, 3, 4) B5H9 : (2302) (3211) (4120) B6H10 :(2402) (3311) (4220) B4H10 :(3103) (4012) B5H11 :(3203) (4112) (5021) B B H B B B B H H B B s ty x (3211) *(3211), the most stable structure which matches well with experimental data Example 3:
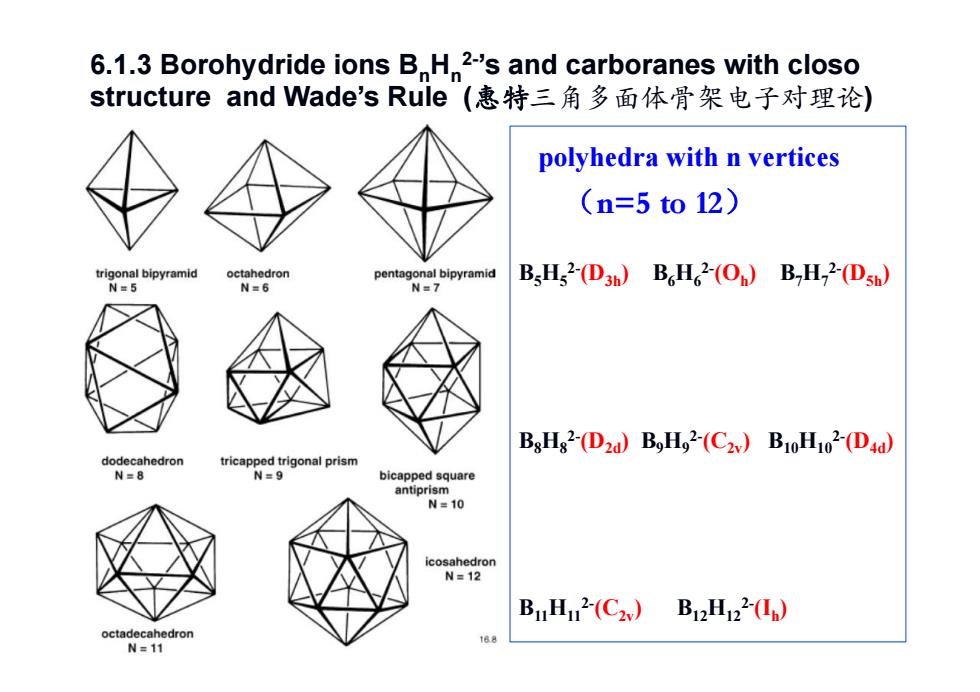
6.1.3 Borohydride ions B H 2-'s and carboranes with closo structure and Wade's Rule(惠特三角多面体骨架电子对理论) polyhedra with n vertices (n=5to12) trigonal bipyramid octahedron pentagonal bipyramid BsHs2-(D3h)B H 2-(On)B-H2-(Dsh) N=5 N=6 N=7 BsHs2-(D2d)BH2-(C2v)B10H102-(Dad) dodecahedron tricapped trigonal prism N=8 N=9 bicapped square antiprism N=10 icosahedron N=12 B1H2(C2) B12H22) octadecahedron N=11
B5H52-(D3h) B6H62-(Oh) B7H72-(D5h) B8H82-(D2d) B9H92-(C2v) B10H102-(D4d) B11H112-(C2v) B12H122-(Ih) polyhedra with n vertices (n=5 to 12) 6.1.3 Borohydride ions BnHn2-’s and carboranes with closo structure and Wade’s Rule (惠特三角多面体骨架电子对理论)