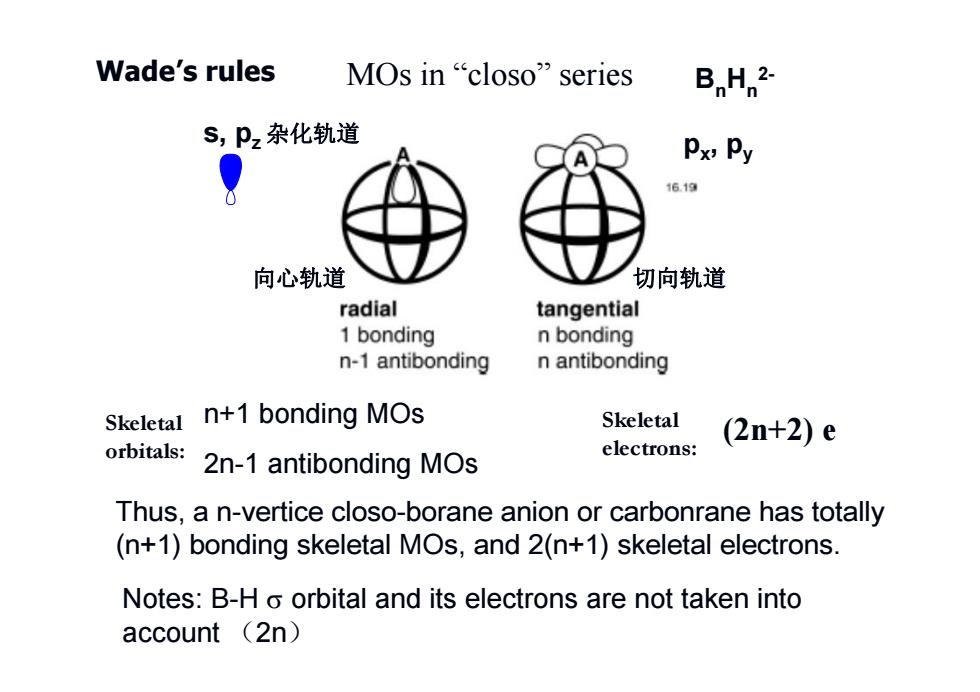
Wade's rules MOs in“closo'series B H 2. S,Pz杂化轨道 Px,Py 16.19 向心轨道 切向轨道 radial tangential 1 bonding n bonding n-1 antibonding n antibonding Skeletal n+1 bonding MOs Skeletal (2n+2)e orbitals: electrons: 2n-1 antibonding MOs Thus,a n-vertice closo-borane anion or carbonrane has totally (n+1)bonding skeletal MOs,and 2(n+1)skeletal electrons. Notes:B-H o orbital and its electrons are not taken into account (2n)
Wade’s rules n+1 bonding MOs 2n-1 antibonding MOs Notes: B-H orbital and its electrons are not taken into account (2n) MOs in “closo” series s, pz 杂化轨道 (2n+2) e px, py Skeletal electrons: BnHn2- Thus, a n-vertice closo-borane anion or carbonrane has totally (n+1) bonding skeletal MOs, and 2(n+1) skeletal electrons. 向心轨道 切向轨道 Skeletal orbitals:

“Closo'”series-formula B H2- Total valence electrons (CVE)=3n (from B)+n (from H) +2 (negative charge)=4n+2 总电子数 Each BH unit uses 2 electrons.Hence skeletal or framework electrons(NFE=4n+2-2n=2n+2) 骨架电子数 Structures are those of the appropriate polyhedra with n vertices There is little tendency to add H+and form neutral species. The closo species are,in effect,the anions of quite strong acids
“Closo” series -formula BnHn2- Total valence electrons (CVE) = 3n (from B) +n (from H) +2 (negative charge) = 4n+2 总电子数 Each BH unit uses 2 electrons. Hence skeletal or framework electrons (NFE=4n+2-2n=2n+2) 骨架电子数 Structures are those of the appropriate polyhedra with n vertices There is little tendency to add H+ and form neutral species. The closo species are , in effect, the anions of quite strong acids

“Nido”series-formula BnH(n+4) Total valence electrons(VEC)=3n (B)+Number of skeletal electrons n(H)+4 (extra H and/or negative charges) to make the structure stable: =4n+4 2n+2 where n=the Framework electrons(NFE)=2n+4. Closo number of surface (2(n+1)+2) atoms present in the cluster,i.e.n =N ·The structure of the“nido”compound is missing surface based on the“closo”polyhedron with one atom on cluster more vertex than the "nido"compound.2n+4 Now N=n+I(so the number of elec- trons 2n'+2 where Nido n'=n+l) "Arachno"series-formula BH(n+6) Total valence electrons(VEC)=4n +6 Framework electrons(NFE)=2n+6 Arachno (2(n+2)+2) 2n+6 Now N=n+2(so the number of elec- ·The structure of the“nido”compound is trons is still 2n"+2 whwere n"=n+2) based on the“closo”polyhedron with two more vertex than the“nido"”compound
“Nido” series – formula B n H(n+4) • Total valence electrons(VEC) = 3n (B) + n(H) + 4 (extra H and/or negative charges) = 4n +4 • Framework electrons (NFE) =2n+4. (2(n+1)+2) • The structure of the “nido” compound is based on the “closo” polyhedron with one more vertex than the “nido” compound. “Arachno” series – formula B n H(n+6) • Total valence electrons(VEC) = 4n +6 • Framework electrons (NFE) =2n+6. (2(n+2)+2) • The structure of the “nido” compound is based on the “closo” polyhedron with two more vertex than the “nido” compound

Examples for Wade's Rule “2n+2 “2n+4” “2n+6” [B。H62 ·BsHg B H10: write as(BH)2 (BH);H3 - Write as(BH)H -Each BH unit 10+4=14 electrons Each BH=>2e (8e in contributes 2e 5 Boron atoms all) Plus the 2-charge bonded by 7 electron 一 Each additional H gives 14 electrons pairs (5+2). gives le (6e in all) 6 boron atoms in the.In terms of electron Total number of cluster bonded with 7 deficiency electrons =14 pairs (6+1). B.H2>BH>B.Hio 4 Borons in cluster 3*6+6+2-2*6 ·All have7 e pairs for bonded by 7 pairs of electrons (4+3). =14e=2*6+2 skeletal bonding (ie cluster bonding). “2n+2” “2n+4” “2n+6
“2n+2” “2n+4” “2n+6” Examples for Wade’s Rule 3*6+6+2-2*6 = 14 e = 2*6+2 “2n+2” “2n+4” “2n+6

General Formula Example Strurctural Type ●[BnHn]P Closo Greek 'closed' (n=6-12) Nido 。[BnHn+4] (n=5,6,8,10,11) Latin‘nest' Aracbno 。[BnHn+6] (=4,5,6,9.) Greek 'spiders web' ·[BnHn+8] Hypbo Not known for simple boron hydrides, (rare) only for adducts,e.g..[B5H9(PMe3)2] Greek 'net
General Formula Example Strurctural Type