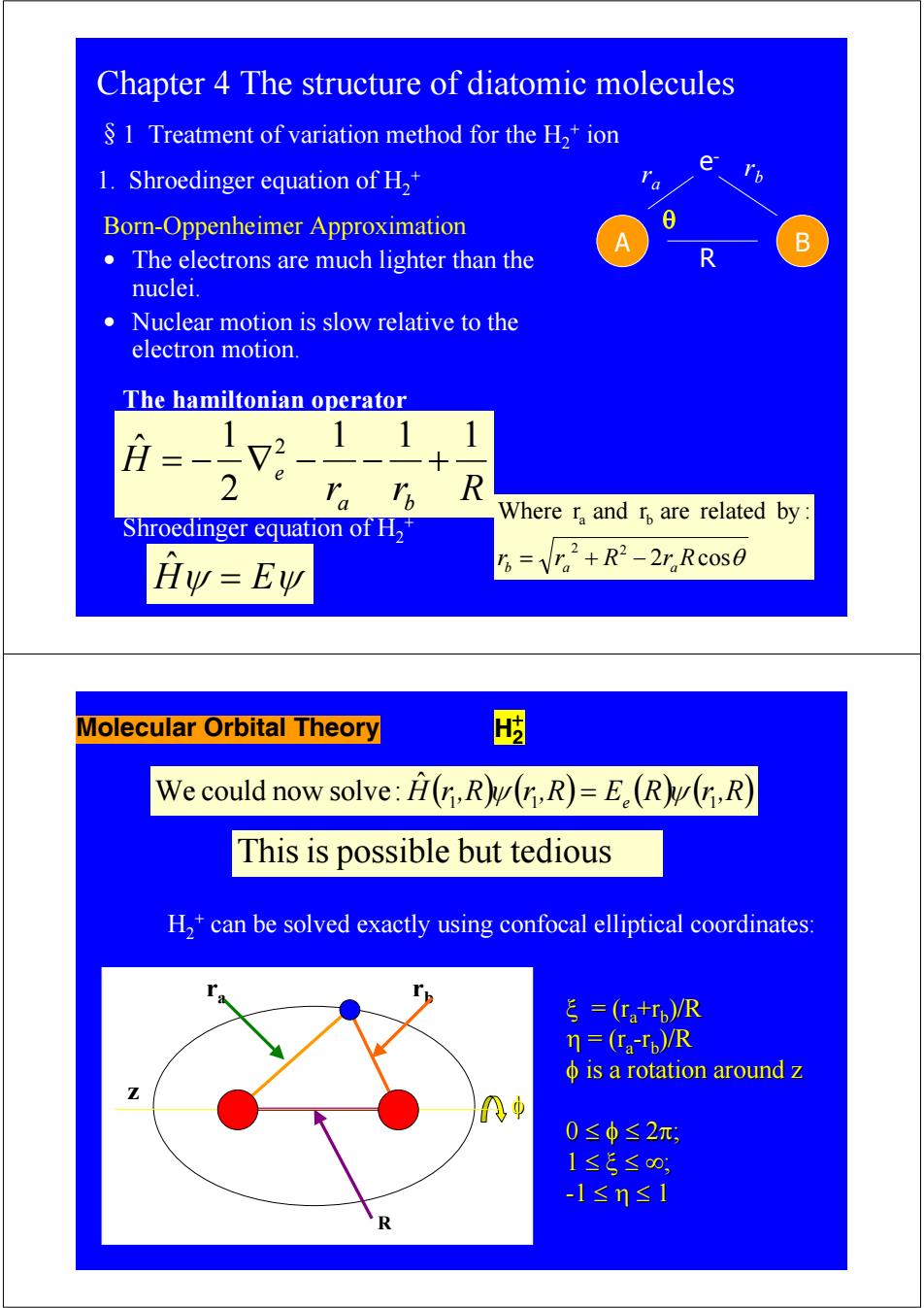
Chapter 4 The structure of diatomic molecules S 1 Treatment of variation method for the H,+ion e 1.Shroedinger equation of H2+ Born-Oppenheimer Approximation 8 B The electrons are much lighter than the nuclei. Nuclear motion is slow relative to the electron motion. The hamiltonian operator 1 1 H 2 、R Where r,and r are related by: Shroedinger equation of H2 Hy=Ew B=V2+R2-2,Rc0s0 Molecular Orbital Theory We could now solve:H(r,R(n,R)=E.(R(r,R) This is possible but tedious H,*can be solved exactly using confocal elliptical coordinates: ξ=(tr,)R n=(ra-Tp)/R φis a rotation around z 0≤φ≤2π, 1≤5≤0, -1≤n≤1 R
Chapter 4 The structure of diatomic molecules §1 Treatment of variation method for the H2 + ion 1. Shroedinger equation of H2 + A B e- r r b a R Born-Oppenheimer Approximation • The electrons are much lighter than the nuclei. • Nuclear motion is slow relative to the electron motion. r r R H a b e 1 1 1 2 1 ˆ 2 = − ∇ − − + The hamiltonian operator 2 cosθ Where r and r are related by : 2 2 a b rb = ra + R − raR θ Hˆψ = Eψ Shroedinger equation of H2 + Molecular Orbital Theory H2 + We could now solve: Hˆ ( )( ) r 1,R ψ r 1,R = Ee (R)( ) ψ r 1,R Thisis possible but tedious H2 + can be solved exactly using confocal elliptical coordinates: ra rb z ξ = (ra +rb)/R η = (ra-rb)/R φ is a rotation around z is a rotation around z 0 ≤ φ ≤ 2π; 1 ≤ ξ ≤ ∞; -1 ≤ η ≤ 1 R φ

Ψe1e=F(5,n)(2元y12eim where m-=0,±l,±2,±3, The associated quantum number is A.>orbital angular momentum 入=m Each electronic level with m 40 is doubly degenerate,i.e.+m,-m atoms:e=0,1.2....and the atomic orbitals are called:s,p,d,etc diatomics:入=0,l,2,.and the molecular orbitals are:o,π,δ,etc 入 letter Vece=F(5,n)(2元-12 eim 入 3 入=ml letter G 元 a e b f (a3
ψelec = F(ξ,η) (2π)-1/2 eimφ where m=0, ±1, ±2, ±3, letter λ σ 0 π 1 δ 2 φ 3 γ 4 The associated quantum number is λ. Æ orbital angular momentum λ =|m| Each electronic level with m ≠0 is doubly degenerate, i.e. + |m|,-|m| atoms: l = 0,1,2,... and the atomic orbitals are called: s,p,d, etc. diatomics: λ = 0,1,2, ... and the molecular orbitals are: σ, π, δ, etc. letter λ σ 0 π 1 δ 2 φ 3 γ 4 ψelec = F(ξ,η) (2π)-1/2 eimφ λ =|m| σ π δ

2.The Variation Theorem For any well-behaved wavefunction the average energy from the Hamiltonian of the system is always greater or close to the exact ground state energy (E)for that Hamiltonian, <E-I96>E0 ∫φdx Example:Devise a trial variation function for the particle in a one-dimensional box of length 1. A simple function that has the properties of the ground state is the parabolic function: p=x(1-x) for0sx≤I 0 =- 6m dr=fx(-xds= 0 <E>- p*idx 5h2 ∫pdr 4r2m12 8ml2
2. The Variation Theorem For any well-behaved wavefunction φ, the average energy from the Hamiltonian of the system is always greater or close to the exact ground state energy (E0) for that Hamiltonian, * 0 * ˆ E d H d E ≥ ∫ ∫ < >= φ φ τ φ φ τ Example: Devise a trial variation function for the particle in a one-dimensional box of length l. 0 l A simple function that has the properties of the ground state is the parabolic function: φ = x(l − x) for 0≤x ≤l m l lx x dx dx d lx x m H d l 6 ( ) ( ) 2 ˆ 2 3 2 2 2 0 2 2 * h h = − − − = ∫ ∫ φ φ τ 30 ( ) 5 2 0 2 * l d x l x dx l = − = ∫ ∫ φ φ τ 2 8 2 2 2 4 2 5 * * ˆ ml h ml h d H d E = ≥ ∫ ∫ < >= φ φ τ π φ φ τ

Proof The Eigenfunctions of an φ(g≥E。)its ground state(w。→Eo) Hermitian operator consist 0=∑cw, of a complete set.V1.V2. 月w,=E, E,之E0 Ψ3.consist of an orthogonal normalized set ∫pidr <E>= of wavefunctions odr fp'dr=∫∑ew,∑cwdr=∑c∑c∫w,y,dr ΣΣcc8,=∑k ∫。idr=∫∑ciwi∑cw,dr=∑ci∑c∫w,iwdr =∑∑ccwE,",dr=∑∑ce,EJw,w,dr=∑kfE ∑e'E, =<E> ≥E。 3.Linear Variation Functions A linear variation function is a linear combination of =cf+c++cnfn=∑cf n linearly independent functions/,2,…a Based on this principle,the parameters are regulated by the minimization routine so as to obtain the wavefunction that 长E-9≥ ∫p*dz corresponds to the minimum energy.This is taken to be the wavefunction that closely 8=<E> approximates the ground state. adjusting the parameter, 08 make =0 aci
j i i j j i j i i j i j i j j i j j i j j i i j j j i i i i i i j ij i j j i j j i i j j j i i i c c E d c c E d c E H d c H c d c c H d c c c d c c d c c d ∑ ∑ ∫ ∑ ∑ ∫ ∑ ∫ ∫ ∑ ∑ ∑ ∑ ∫ ∑ ∑ ∑ ∫ ∫ ∑ ∑ ∑ ∑ ∫ = = = = = = = * * 2 * * * * * 2 * * * * * ˆ ˆ ˆ ψ ψ τ ψ ψ τ φ φ τ ψ ψ τ ψ ψ τ δ φ φ τ= ψ ψ τ= ψ ψ τ The Eigenfunctions of an Hermitian operator consist of a complete set. ψ1, ψ2, ψ3 …consist of an orthogonal normalized set of wavefunctions φ φ τ φ φ τ ψ ψ φ ψ φ ε ψ d H d E H E E E c E ground state E i i i i i i i ∫ ∫ ∑ < >= = ≥ = ≥ → * * 0 0 0 0 ˆ ˆ ( ) its ( ) Proof 2 0 2 =< >= ≥ E ∑ ∑ i i j i i c c E ε E adjusting the parameter, make = 0 ∂ ∂ i c ε Based on this principle, the parameters are regulated by the minimization routine so as to obtain the wavefunction that corresponds to the minimum energy. This is taken to be the wavefunction that closely approximates the ground state. * 0 * ˆ E d H d E ≥ ∫ ∫ < >= φ φ τ φ φ τ ε =< E > 3. Linear Variation Functions ∑= = + + + = n j n n j j c f c f c f c f 1 1 1 2 2 φ ... A linear variation function is a linear combination of n linearly independent functions f1, f2, …fn

Example [中Hdr =C4+C2Ψ2 8= odr 「p'dr=「(cy+c4)'(c4+c22)dr =∫(G244+cc242+cc44+c222)dz =」(G44+2cc42+c42)dt =c2+2cc,S2+622 (Sy=∫4iy,dr=S) =c12S1+2c0S2+c22S2 (S1=S22=1) 「padr=c4tc,y)广a(c4+c业)dr (c'vHy+ccz+cc2 Hy+cW:HV2)dt CHn+2cc2Hn+c2Hz (H,=Hn=∫4iy,) (Hm=H2=1) let c'Hn+2cc,Hn+c'Hny c2S1+2cc2S2+622S2 X make 6→Eo 0= O8 1 dy y ox ac x 0c x2 0c (2cH+26,H2)-2GS+26,Sa) 2cH+2c,.)-S+2cc.+c2(2c5+2c,5)=0 CSu+2cC2S2+C2S2 (2cH1,+2c2H12)-E(2cS1+2c2S12)=0 (cH1+c2H2)-E(cS1+c2S2)=0 (Hu-ES])c +(H12-ES2)c2=0 () same as 0= OE OC2 (H21-ES21)G1+(H2-ES2)c2=0 (2)
( 1) ) ˆ 2 ( ) ˆ ˆ ˆ ˆ ( ( ) ˆ ( ) ˆ 11 22 * 22 2 11 1 2 12 2 2 1 2 * 2 2 1 2 * 2 1 2 2 * 1 1 2 1 * 1 2 1 1 1 2 2 * 1 1 2 2 * = = = + + = = = + + + = + + ∫ ∫ ∫ ∫ H H c H c c H c H H H H c H c c H c c H c H d H d c c H c c d ij ji ψ i ψ j ψ ψ ψ ψ ψ ψ ψ ψ τ φ φ τ ψ ψ ψ ψ τ Example 2 ( 1) 2 ( ) ( 2 ) ( ) ( ) ( ) 22 11 22 2 11 1 2 12 2 2 1 ji * 1 2 1 2 12 2 2 1 2 * 2 2 2 2 * 1 1 2 1 * 1 2 1 2 * 2 2 1 2 * 2 1 2 2 * 1 1 2 1 * 1 2 1 1 1 2 2 * 1 1 2 2 * = + + = = = + + = = + + = + + + = + + ∫ ∫ ∫ ∫ ∫ c S c c S c S S S c c c S c S d S c c c c d c c c c c c d d c c c c d ij ψ ψ j τ= ψ ψ ψ ψ ψ ψ τ ψ ψ ψ ψ ψ ψ ψ ψ τ φ φ τ ψ ψ ψ ψ τ φ φ τ φ φ τ φ ψ ψ ε d H d c c ∫ ∫ = + = * * 1 1 2 2 ˆ ( ) ( ) 0 (2) 0 21 21 1 22 22 2 2 − + − = ∂ ∂ = H ES c H ES c c same as ε ( ) ( ) 0 (1) ( ) ( ) 0 (2 2 ) (2 2 ) 0 (2 2 ) 0 2 2 (2 2 ) (2 2 ) (2 2 ) 1 1 0 11 11 1 12 12 2 1 11 2 12 1 11 2 12 1 11 2 12 1 11 2 12 1 11 2 12 22 2 11 1 2 12 2 2 1 22 2 11 1 2 12 2 2 1 1 11 2 12 1 11 2 12 2 1 11 2 12 1 2 1 1 0 − + − = + − + = + − + = + = + + + + + − = + − + ∂ ∂ − ∂ ∂ = ∂ ∂ = ⇒ H ES c H ES c c H c H E c S c S c H c H E c S c S c S c S c S c c S c S c H c c H c H c H c H c S c S x y c H c H x c x x y c y c x make E ε ε x y c S c c S c S c H c c H c H let = + + + + = 22 2 11 1 2 12 2 2 1 22 2 11 1 2 12 2 2 1 2 2 ε