
Chapter 7 Introduction to Crystallography Diamonds
Chapter 7 Chapter 7 Introduction to Introduction to Crystallography Crystallography Diamonds
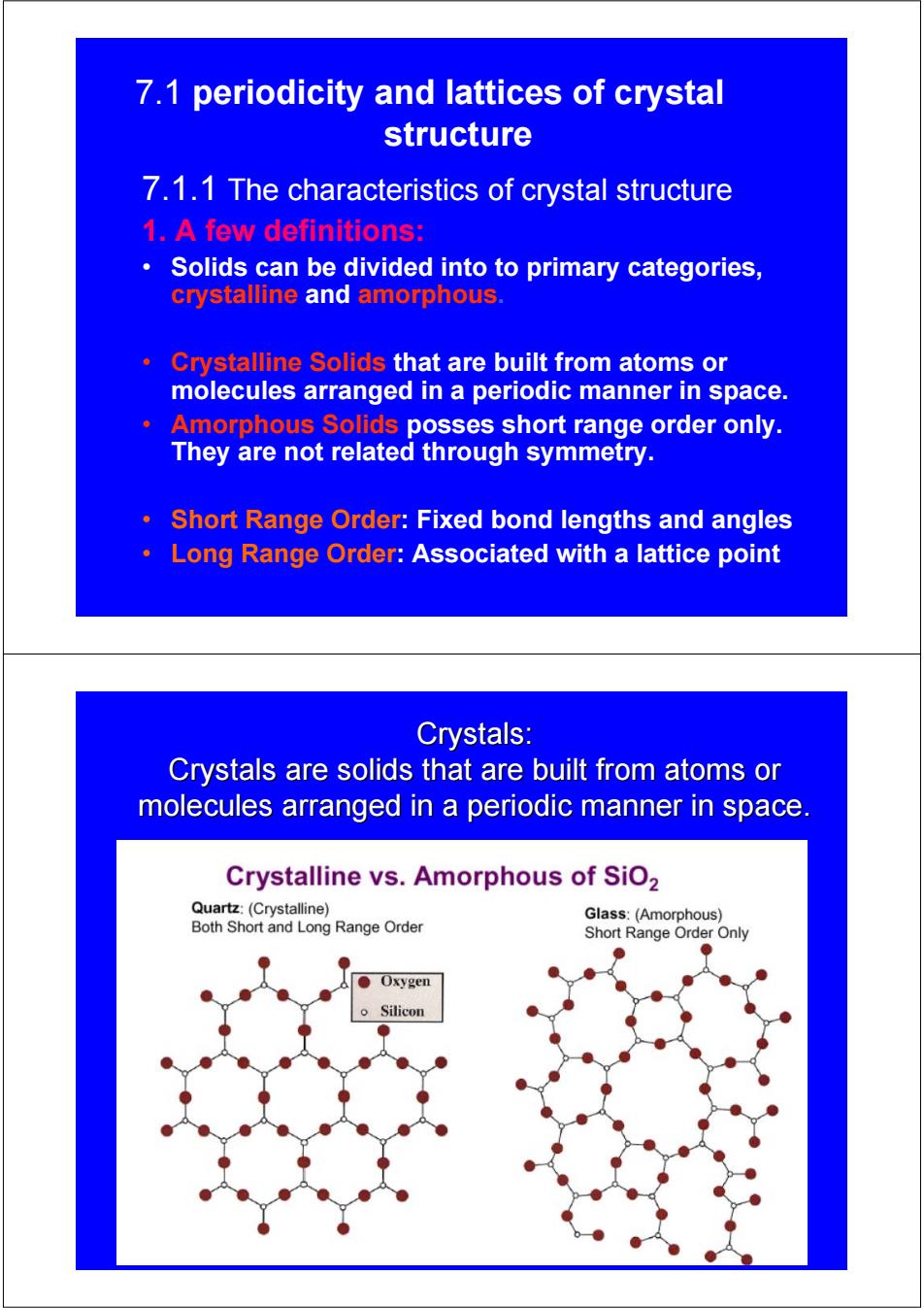
7.1 periodicity and lattices of crystal structure 7.1.1 The characteristics of crystal structure 1.A few definitions: Solids can be divided into to primary categories, crystalline and amorphous. Crystalline Solids that are built from atoms or molecules arranged in a periodic manner in space. Amorphous Solids posses short range order only. They are not related through symmetry. Short Range Order:Fixed bond lengths and angles Long Range Order:Associated with a lattice point Crystals: Crystals are solids that are built from atoms or molecules arranged in a periodic manner in space. Crystalline vs.Amorphous of SiO2 Quartz:(Crystalline) Glass:(Amorphous) Both Short and Long Range Order Short Range Order Only Oxygen Silicon
7.1 periodicity and lattices of crystal structure 7.1.1 The characteristics of crystal structure 1. A few definitions: • Solids can be divided into to primary categories, crystalline and amorphous. • Crystalline Solids that are built from atoms or molecules arranged in a periodic manner in space. • Amorphous Solids posses short range order only. They are not related through symmetry. • Short Range Order: Fixed bond lengths and angles • Long Range Order: Associated with a lattice point Crystals: Crystals: Crystals are solids that are built from atoms or Crystals are solids that are built from atoms or molecules arranged in a periodic manner in space. molecules arranged in a periodic manner in space
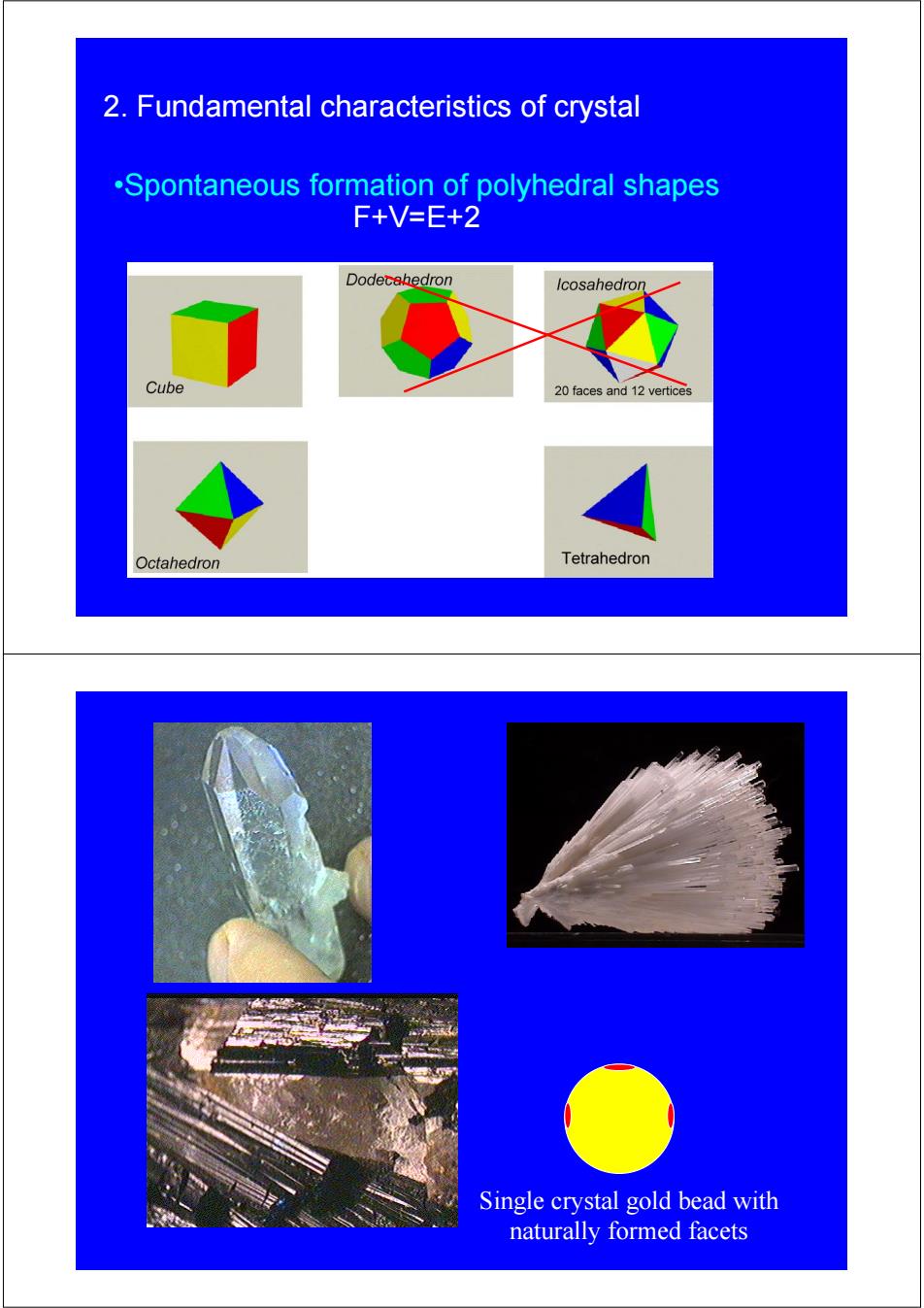
2.Fundamental characteristics of crystal .Spontaneous formation of polyhedral shapes F+V=E+2 Dodecahedron Icosahedron Cube 20 faces and 12 vertices Octahedron Tetrahedron Single crystal gold bead with naturally formed facets
2. Fundamental characteristics of crystal •Spontaneous formation of polyhedral shapes F+V=E+2 Single crystal gold bead with naturally formed facets
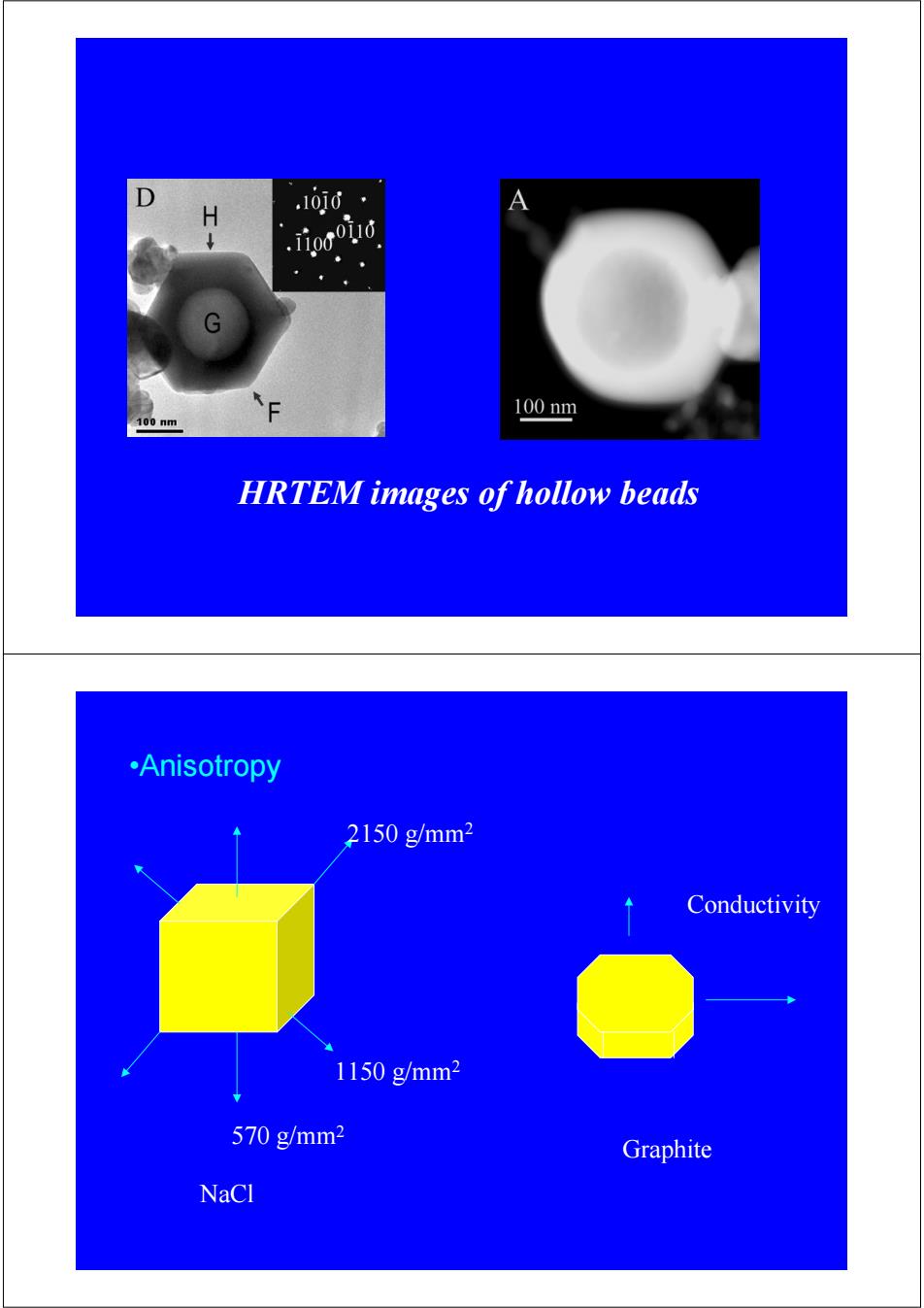
H 1010· .rooind 100nm 100nm HRTEM images of hollow beads •Anisotropy 2150g/mm2 Conductivity 1150g/mm2 570g/mm2 Graphite NaCl
HRTEM images of hollow beads •Anisotropy NaCl 570 g/mm2 1150 g/mm2 2150 g/mm2 Graphite Conductivity
.Definite sharp melting points ·Symmetry -X-ray diffraction by crystals 7.1.2 The lattice and unit cell ·Lattice: A periodic pattern of points in space,such that each lattice point has identical surroundings. 。 Can be reproduced by translational motion along the vector between any two points
• Symmetry •Definite sharp melting points •X-ray diffraction by crystals t T 7.1.2 The lattice and unit cell • Lattice: • A periodic pattern of points in space, such that each lattice point has identical surroundings. • Can be reproduced by translational motion along the vector between any two points