
Slater orbitals(STOs) A Slater function centered on nucleus a has the form X=Nra-eYm(0) (8.5) Molecular orbital (MO) 4=∑cn (8.6) where the 's are the STO basis functions.We have LC-STO MOs. For polyatomic molecules,the LC-STO method uses STOs centered on each of the atoms.The presence of more than two atoms causes difficulties in evaluating the needed integrals Gaussian-type orbitals(GTOs) Computer evaluation of three-and four-center integrals over STO basis functions is very time consuming.To simplify molecular integral evaluation,Boys proposed in 1950 the use of Gaussian-type functions (GTOs)instead of STOs for the atomic orbitals in an LCAO wave function. A Cartesian Gaussian centered on nucleus a is defined as 8i=x。yze (8.7) where N is the normalization constant,i,j,and k are nonnegative integers, and a is positive orbital exponent. i+j+k=0,s-type Gaussian itj+k=1,p-type Gaussians,px pP: i+j+k=2,d-type Gaussians,6 d-type Gaussians, with the factors x2,y,z,xy,xz,yz.They can form five linear combinations having the same angular behavior as the five 3d AOs;the sixth combination with the factor x2+y2+z2=r2 is like a 3s AO
Slater orbitals (STOs) A Slater function centered on nucleus a has the form ( , ) a a m l n r r Nra e Y a χ θ φ − −ξ = 1 (8.5) Molecular orbital (MO) r r i ri φ = ∑ c χ (8.6) where the χr’s are the STO basis functions. We have LC-STO MOs. For polyatomic molecules, the LC-STO method uses STOs centered on each of the atoms. The presence of more than two atoms causes difficulties in evaluating the needed integrals. Gaussian-type orbitals (GTOs) Computer evaluation of three- and four-center integrals over STO basis functions is very time consuming. To simplify molecular integral evaluation, Boys proposed in 1950 the use of Gaussian-type functions (GTOs) instead of STOs for the atomic orbitals in an LCAO wave function. A Cartesian Gaussian centered on nucleus a is defined as 2 a k r a j a i ijk a g Nx y z e −α = (8.7) where N is the normalization constant, i, j, and k are nonnegative integers, and α is positive orbital exponent. i+j+k = 0, s-type Gaussian i+j+k = 1, p-type Gaussians, px, py, pz. i+j+k = 2, d-type Gaussians, 6 d-type Gaussians, with the factors x2 , y 2 , z 2 , xy, xz, yz. They can form five linear combinations having the same angular behavior as the five 3d AOs; the sixth combination with the factor x 2 + y2 + z2 = r 2 is like a 3s AO
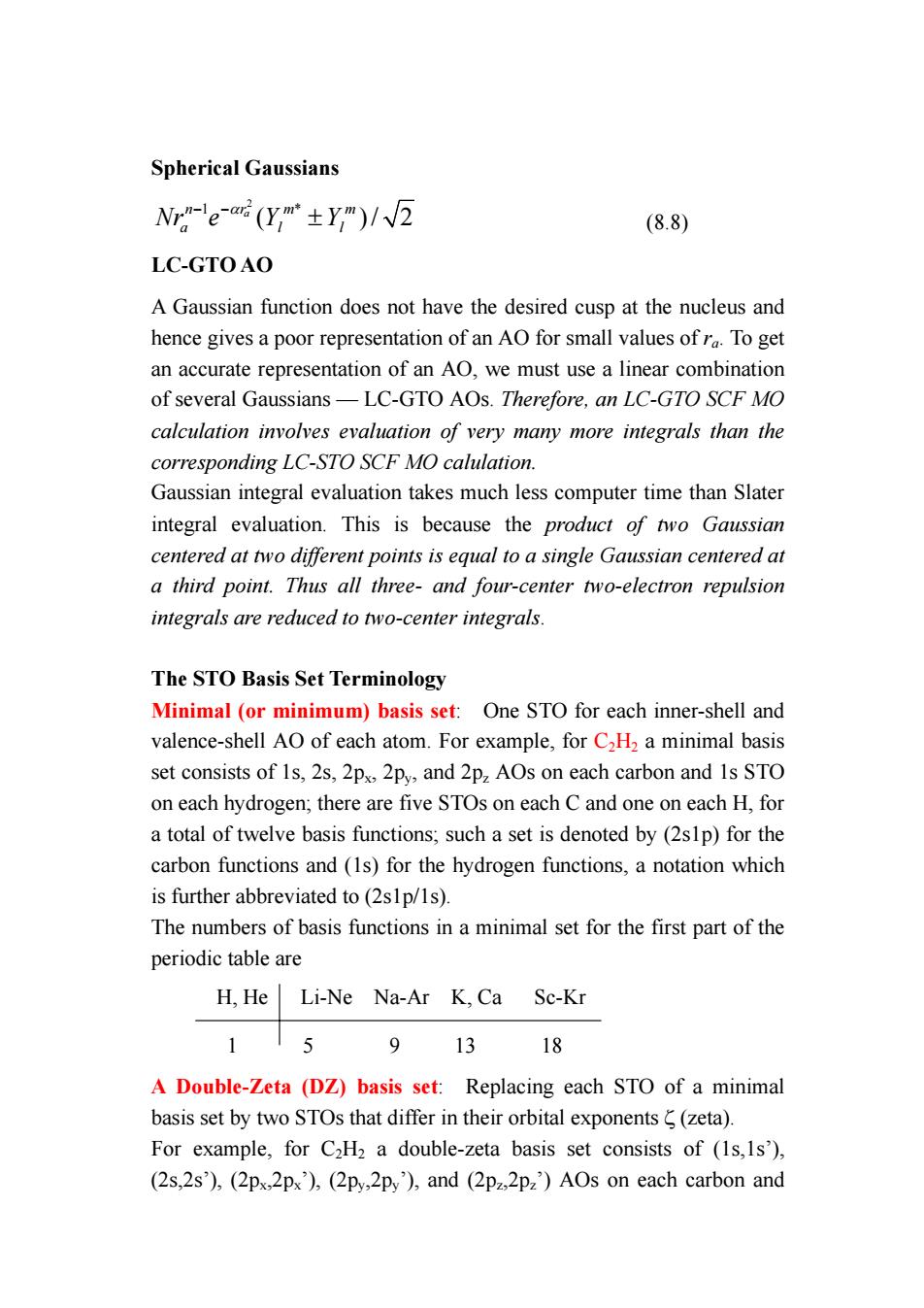
Spherical Gaussians Nr-eaG(y,m±ym)/W2 (8.8) LC-GTOAO A Gaussian function does not have the desired cusp at the nucleus and hence gives a poor representation of an AO for small values ofr To get an accurate representation of an AO,we must use a linear combination of several Gaussians-LC-GTO AOs.Therefore,an LC-GTO SCF MO calculation involves evaluation of very many more integrals than the corresponding LC-STO SCF MO calulation. Gaussian integral evaluation takes much less computer time than Slater integral evaluation.This is because the product of two Gaussian centered at two different points is equal to a single Gaussian centered at a third point.Thus all three-and four-center two-electron repulsion integrals are reduced to two-center integrals. The STO Basis Set Terminology Minimal (or minimum)basis set:One STO for each inner-shell and valence-shell AO of each atom.For example,for C2H2 a minimal basis set consists of 1s,2s,2p 2py,and 2p AOs on each carbon and Is STO on each hydrogen,there are five STOs on each C and one on each H,for a total of twelve basis functions,such a set is denoted by(2slp)for the carbon functions and (1s)for the hydrogen functions,a notation which is further abbreviated to(2s1p/1s). The numbers of basis functions in a minimal set for the first part of the periodic table are H,He Li-Ne Na-Ar K,Ca Sc-Kr 115 91318 A Double-Zeta (DZ)basis set:Replacing each STO of a minimal basis set by two STOs that differ in their orbital exponents(zeta). For example,for C2H2 a double-zeta basis set consists of (1s,Is'), (2s,2s),(2px,2px),(2py,2p),and (2p22p2)AOs on each carbon and
Spherical Gaussians 2 1 * ( )/ 2 a n mm r Nr e Y Y a ll − −α ± (8.8) LC-GTO AO A Gaussian function does not have the desired cusp at the nucleus and hence gives a poor representation of an AO for small values of ra. To get an accurate representation of an AO, we must use a linear combination of several Gaussians — LC-GTO AOs. Therefore, an LC-GTO SCF MO calculation involves evaluation of very many more integrals than the corresponding LC-STO SCF MO calulation. Gaussian integral evaluation takes much less computer time than Slater integral evaluation. This is because the product of two Gaussian centered at two different points is equal to a single Gaussian centered at a third point. Thus all three- and four-center two-electron repulsion integrals are reduced to two-center integrals. The STO Basis Set Terminology Minimal (or minimum) basis set: One STO for each inner-shell and valence-shell AO of each atom. For example, for C2H2 a minimal basis set consists of 1s, 2s, 2px, 2py, and 2pz AOs on each carbon and 1s STO on each hydrogen; there are five STOs on each C and one on each H, for a total of twelve basis functions; such a set is denoted by (2s1p) for the carbon functions and (1s) for the hydrogen functions, a notation which is further abbreviated to (2s1p/1s). The numbers of basis functions in a minimal set for the first part of the periodic table are H, He Li-Ne Na-Ar K, Ca Sc-Kr 1 5 9 13 18 A Double-Zeta (DZ) basis set: Replacing each STO of a minimal basis set by two STOs that differ in their orbital exponents ζ (zeta). For example, for C2H2 a double-zeta basis set consists of (1s,1s’), (2s,2s’), (2px,2px’), (2py,2py’), and (2pz,2pz’) AOs on each carbon and