
Symmetric Molecules CH3 CH3 COOH COOH H Br Br H H- OH H OH H Br H Br H OH HO H CH3 CH3 COOH COOH meso (±)or(d,l) meso (±)or(d,l) 2,3-dibromobutane tartaric acid Copyright2010 Pearson Prentice Hall,Inc. Erythro and threo are not used on molecules with similar ends. For symmetric molecules,the terms meso and (d,/)are used. Chapter 23 11
Chapter 23 11 Symmetric Molecules ▪ Erythro and threo are not used on molecules with similar ends. ▪ For symmetric molecules, the terms meso and (d,l) are used
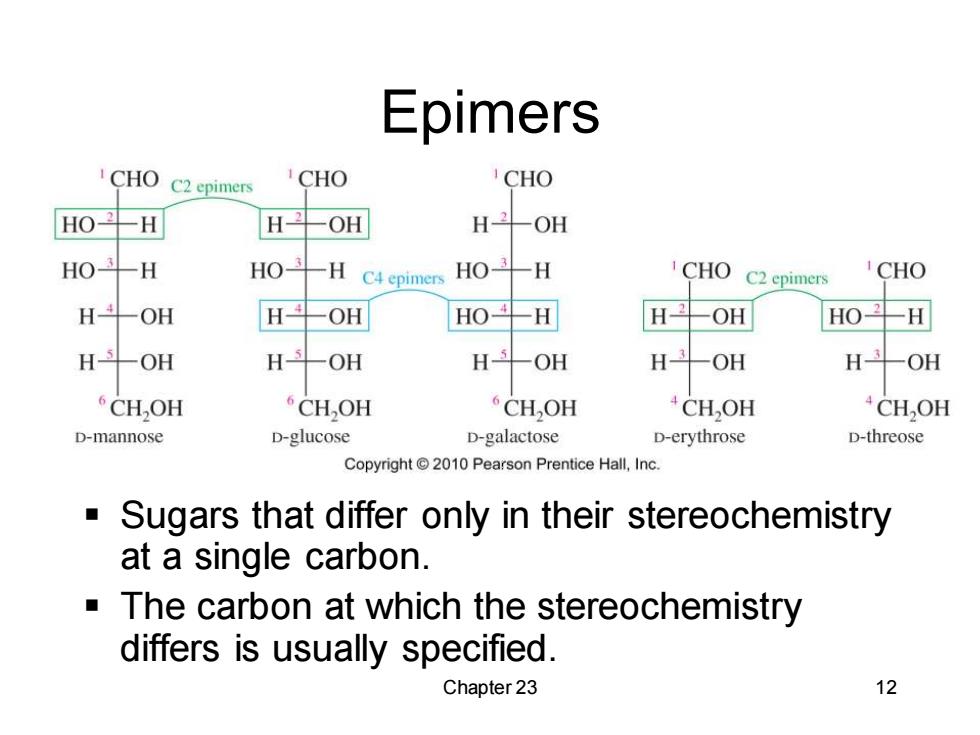
Epimers CHO C2 epimers CHO CHO HO H H2OH H-OH HO斗H HO H C4 epimers HO-H CHO )C2 epimers CHO H-OH H--OH HOH H--OH HO- H H-OH H-OH H-OH H--OH H- -OH CH,OH CH,OH CH,OH CH,OH CH,OH D-mannose D-glucose D-galactose D-erythrose D-threose Copyright 2010 Pearson Prentice Hall,Inc. Sugars that differ only in their stereochemistry at a single carbon. The carbon at which the stereochemistry differs is usually specified. Chapter 23 12
Chapter 23 12 Epimers ▪ Sugars that differ only in their stereochemistry at a single carbon. ▪ The carbon at which the stereochemistry differs is usually specified
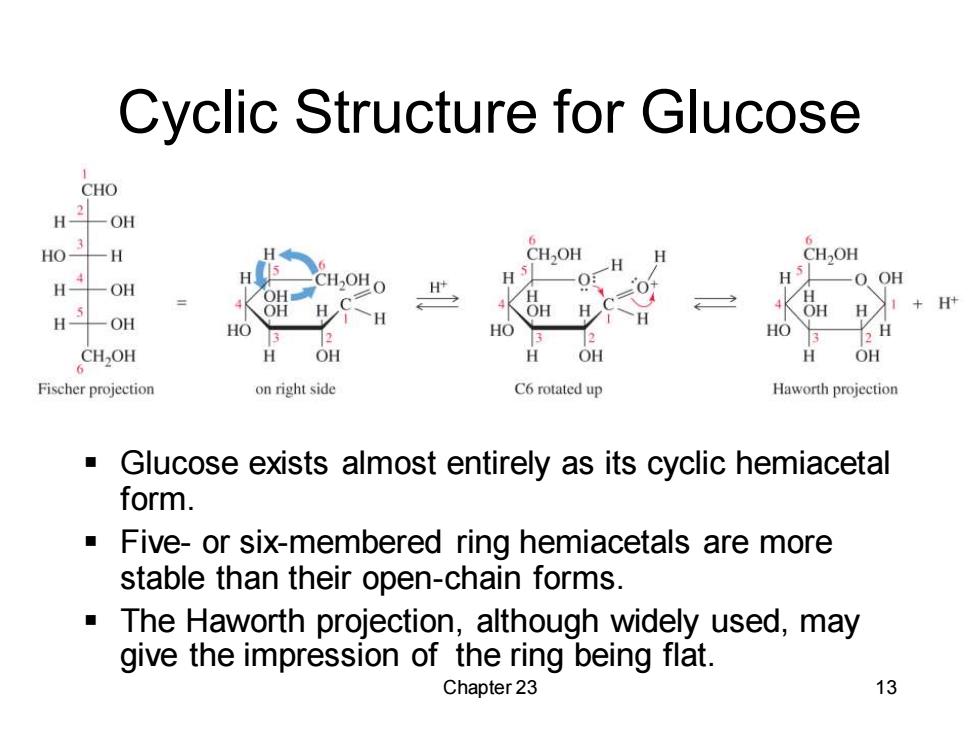
Cyclic Structure for Glucose CHO -OH H03 6 -H CH,OH H OH H CH-OHO H H H O OH -OH H 4K OH H 义1+Ht 一OH H HO HO HO 3 H 。CH,0H OH OH 分 OH Fischer projection on right side C6 rotated up Haworth projection ■ Glucose exists almost entirely as its cyclic hemiacetal form. Five-or six-membered ring hemiacetals are more stable than their open-chain forms. The Haworth projection,although widely used,may give the impression of the ring being flat. Chapter 23 13
Chapter 23 13 Cyclic Structure for Glucose ▪ Glucose exists almost entirely as its cyclic hemiacetal form. ▪ Five- or six-membered ring hemiacetals are more stable than their open-chain forms. ▪ The Haworth projection, although widely used, may give the impression of the ring being flat

Chair Conformation for Glucose H CH2OH CI is the only carbon atom CH,OH HO4 5 bonded to 2 oxygens HO 5 H H HO 2 OH HO H -H 3 OH O H H H OH chair conformation(all substituents equatorial) chair conformation (OH on CI axial) Copyright 2010 Pearson Prentice Hall,Inc The chair conformations give a more accurate representation of glucose. Glucose exists almost entirely as its cyclic hemiacetal form. Chapter 23 14
Chapter 23 14 Chair Conformation for Glucose ▪ The chair conformations give a more accurate representation of glucose. ▪ Glucose exists almost entirely as its cyclic hemiacetal form

Cyclic Structure for Fructose ICH2OH 2C=0 HO-3-H HO-CH2Q 0-H HOCH20 OH KH HOZ H HO H-4OH H4 CH,OH H4 3 CH,OH H-5-OH OH H OH H cyclic form 6CH,OH D-fructose Copyright 2010 Pearson Prentice Hall,Inc. Cyclic hemiacetal formed by reaction of C=O at C2 with -OH at C5. Since five-membered rings are not puckered as much as six-membered rings,they are usually depicted as flat Haworth projections. Chapter 23 15
Chapter 23 15 Cyclic Structure for Fructose ▪ Cyclic hemiacetal formed by reaction of C═O at C2 with —OH at C5. ▪ Since five-membered rings are not puckered as much as six-membered rings, they are usually depicted as flat Haworth projections