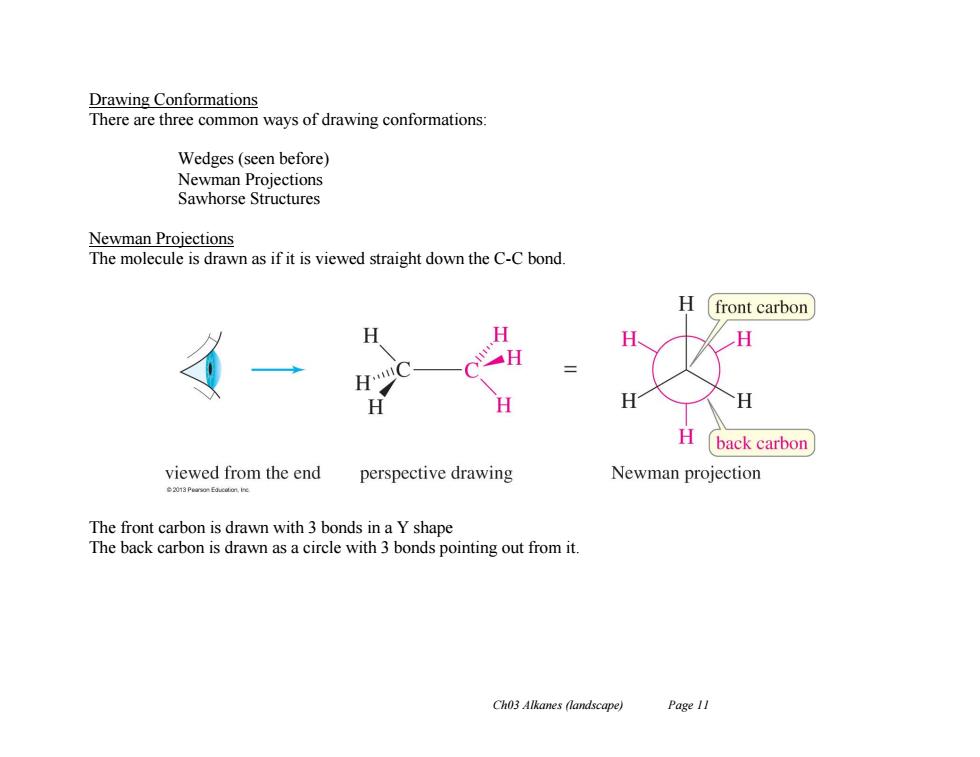
Drawing Conformations There are three common ways of drawing conformations: Wedges(seen before) Newman Projections Sawhorse Structures Newman Projections The molecule is drawn as if it is viewed straight down the C-C bond. H front carbon H H back carbon viewed from the end perspective drawing Newman projection The front carbon is drawn with 3 bonds in a Y shape The back carbon is drawn as a circle with 3 bonds pointing out from it. Ch03 Alkanes (landscape) Page 11
Ch03 Alkanes (landscape) Page 11 Drawing Conformations There are three common ways of drawing conformations: Wedges (seen before) Newman Projections Sawhorse Structures Newman Projections The molecule is drawn as if it is viewed straight down the C-C bond. The front carbon is drawn with 3 bonds in a Y shape The back carbon is drawn as a circle with 3 bonds pointing out from it

Sawhorse Structures These picture the molecule as viewed looking down on the C-C bond at an angle from above. H H H H H H H H H 夕 H H H eclipsed,=0 staggered,0=60 skew,0=anything else 21移h与达65gne Newman projections: 0=0 HH H=60 H H The dihedral angle,0,is the angle between the C-H bonds on the front of a Newman projection,and those on the back. When 0=0 it is known as the Eclipsed conformation When 0=60 it is called the Staggered conformation. Any other conformation is known as a Skew conformation. Ch03 Alkanes (landscape) Page 12
Ch03 Alkanes (landscape) Page 12 Sawhorse Structures These picture the molecule as viewed looking down on the C-C bond at an angle from above. The dihedral angle, , is the angle between the C-H bonds on the front of a Newman projection, and those on the back. When = 0° it is known as the Eclipsed conformation. When = 60° it is called the Staggered conformation. Any other conformation is known as a Skew conformation

The energy difference between these conformations is only about 3kcal/mol (13kJ/mol).This is a small amount of energy,and at room temperature,molecules have enough energy to overcome this small barrier. Therefore,a room temperature sample of ethane gas would contain all the different conformations-although not all in the same proportions The energy difference arises from electron repulsions between the different C-H bond electrons. kcal/mo 12.6kJ/m0l0 120 dihedral angle 志字名 As the ethane molecule rotates through eclipsed and staggered conformations,the potential energy of the systems changes. The resistance to rotation is called torsional strain,and the 3kcal/mol is called the torsional energy Many organic reactions depend on a molecule's conformation,and the study of this is called conformational analysis. Ch03 Alkanes (landscape) Page 13
Ch03 Alkanes (landscape) Page 13 The energy difference between these conformations is only about 3kcal/mol (13kJ/mol). This is a small amount of energy, and at room temperature, molecules have enough energy to overcome this small barrier. Therefore, a room temperature sample of ethane gas would contain all the different conformations – although not all in the same proportions. The energy difference arises from electron repulsions between the different C-H bond electrons. As the ethane molecule rotates through eclipsed and staggered conformations, the potential energy of the systems changes. The resistance to rotation is called torsional strain, and the 3kcal/mol is called the torsional energy. Many organic reactions depend on a molecule’s conformation, and the study of this is called conformational analysis