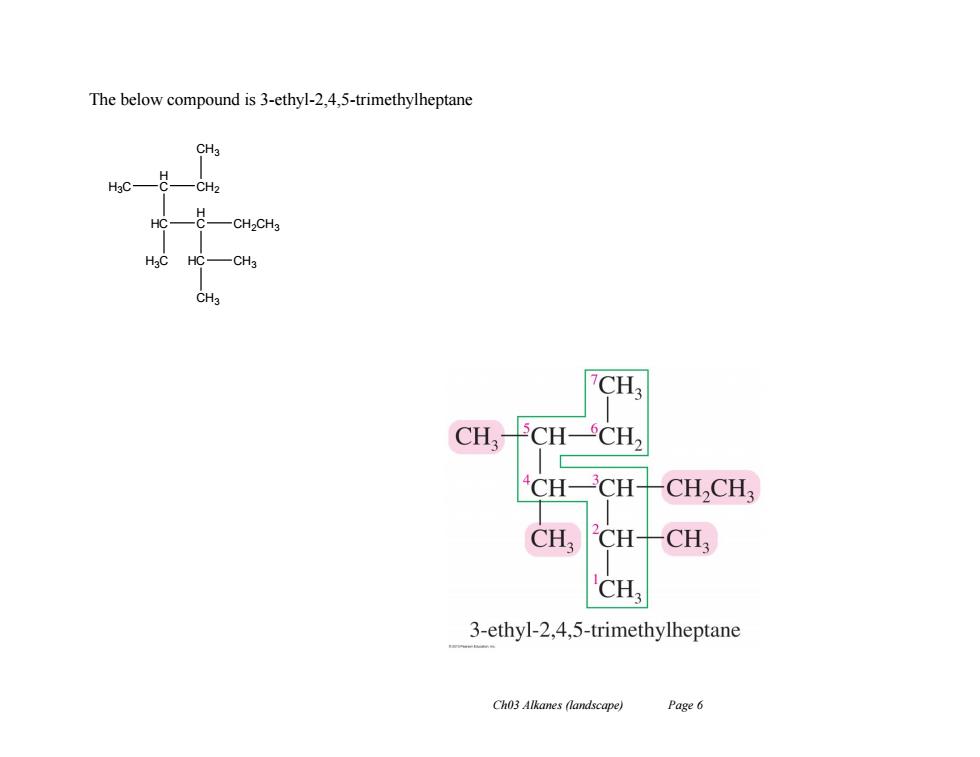
The below compound is 3-ethyl-2,4,5-trimethylheptane CH3 H3C -CH2 HC -CH2CHg CH3 CH3 CH,- CH-CH2 CH-CH- CH,CH3 CH CH- CH CH 3-ethyl-2,4,5-trimethylheptane Ch03 Alkanes (landscape) Page 6
Ch03 Alkanes (landscape) Page 6 The below compound is 3-ethyl-2,4,5-trimethylheptane CH3 CH2 H C HC H3C H C H3C HC CH2CH3 CH3 CH3

Complex Substituents These are named as follows: (a)The base alkyl group is numbered starting with the carbon bonded to the main chain. (b) The substituents are listed with the appropriate numbers,and parentheses are used to separate the substituent name. CH3CH3 -C-CH-CH-CH3 CH3 a(1,1,3-trimethylbutyl)group Properties of Alkanes Natural gas,gasoline,oils and paraffin wax are all alkanes,and so alkanes are often used as fuels,lubricants and solvents. Alkanes are non-polar,and are said to be Hydrophobic(water hating)since they do not dissolve in water. Typically the density of alkanes is around 0.7g/ml,and so when an alkane and water are mixed,they will form two separate phases,with the alkane on top.(Oil floats on water). Ch03 Alkanes (landscape) Page 7
Ch03 Alkanes (landscape) Page 7 Complex Substituents These are named as follows: (a) The base alkyl group is numbered starting with the carbon bonded to the main chain. (b) The substituents are listed with the appropriate numbers, and parentheses are used to separate the substituent name. Properties of Alkanes Natural gas, gasoline, oils and paraffin wax are all alkanes, and so alkanes are often used as fuels, lubricants and solvents. Alkanes are non-polar, and are said to be Hydrophobic (‘water hating’) since they do not dissolve in water. Typically the density of alkanes is around 0.7g/ml, and so when an alkane and water are mixed, they will form two separate phases, with the alkane on top. (Oil floats on water). CH3 C-CH2 CH3 CH-CH3 CH3 a (1,1,3-trimethylbutyl) group
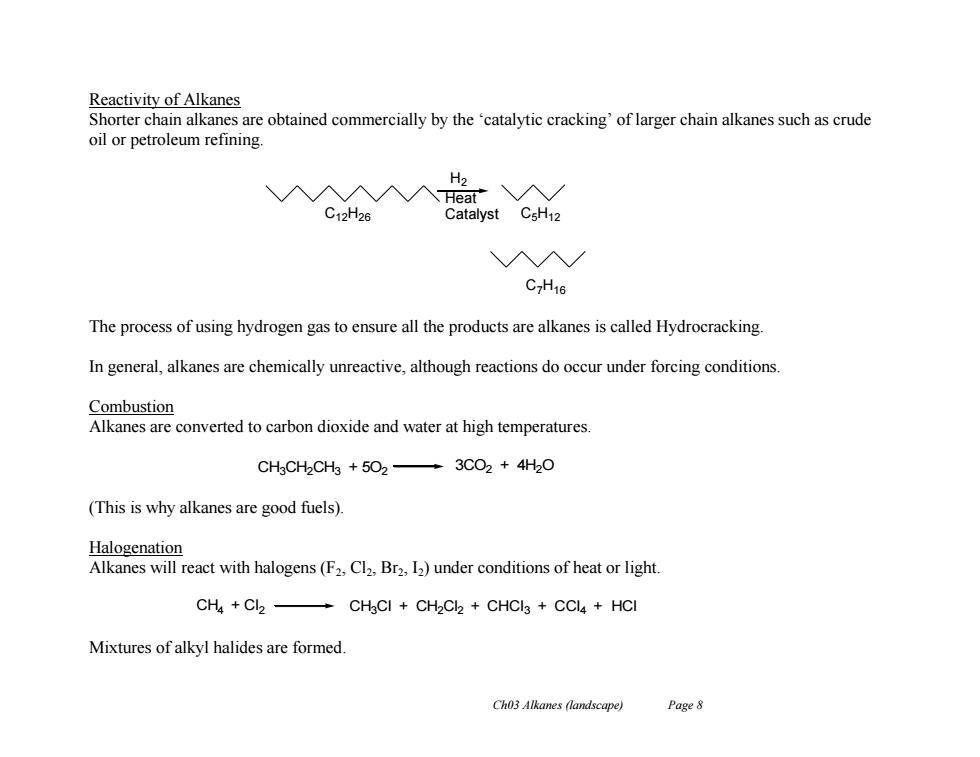
Reactivity of Alkanes Shorter chain alkanes are obtained commercially by the 'catalytic cracking'of larger chain alkanes such as crude oil or petroleum refining H2 入A入入Heat"入/ C12H26 Catalyst CsH12 入N/ C7H16 The process of using hydrogen gas to ensure all the products are alkanes is called Hydrocracking. In general,alkanes are chemically unreactive,although reactions do occur under forcing conditions Combustion Alkanes are converted to carbon dioxide and water at high temperatures. CHgCH2CH3 +502-3CO2 4H2O (This is why alkanes are good fuels). Halogenation Alkanes will react with halogens(F2,Cl2,Br2,12)under conditions of heat or light. CH4 Cl2- CH3Cl CH2Cl2 CHCI3 CCl4 HCI Mixtures of alkyl halides are formed. Ch03 Alkanes (landscape) Page 8
Ch03 Alkanes (landscape) Page 8 Reactivity of Alkanes Shorter chain alkanes are obtained commercially by the ‘catalytic cracking’ of larger chain alkanes such as crude oil or petroleum refining. The process of using hydrogen gas to ensure all the products are alkanes is called Hydrocracking. In general, alkanes are chemically unreactive, although reactions do occur under forcing conditions. Combustion Alkanes are converted to carbon dioxide and water at high temperatures. (This is why alkanes are good fuels). Halogenation Alkanes will react with halogens (F2, Cl2, Br2, I2) under conditions of heat or light. Mixtures of alkyl halides are formed. H2 Heat C12H26 Catalyst C5H12 C7H16 CH4 + Cl2 CH3Cl + CH2Cl2 + CHCl3 + CCl4 + HCl CH3CH2CH3 + 5O2 3CO2 + 4H2O
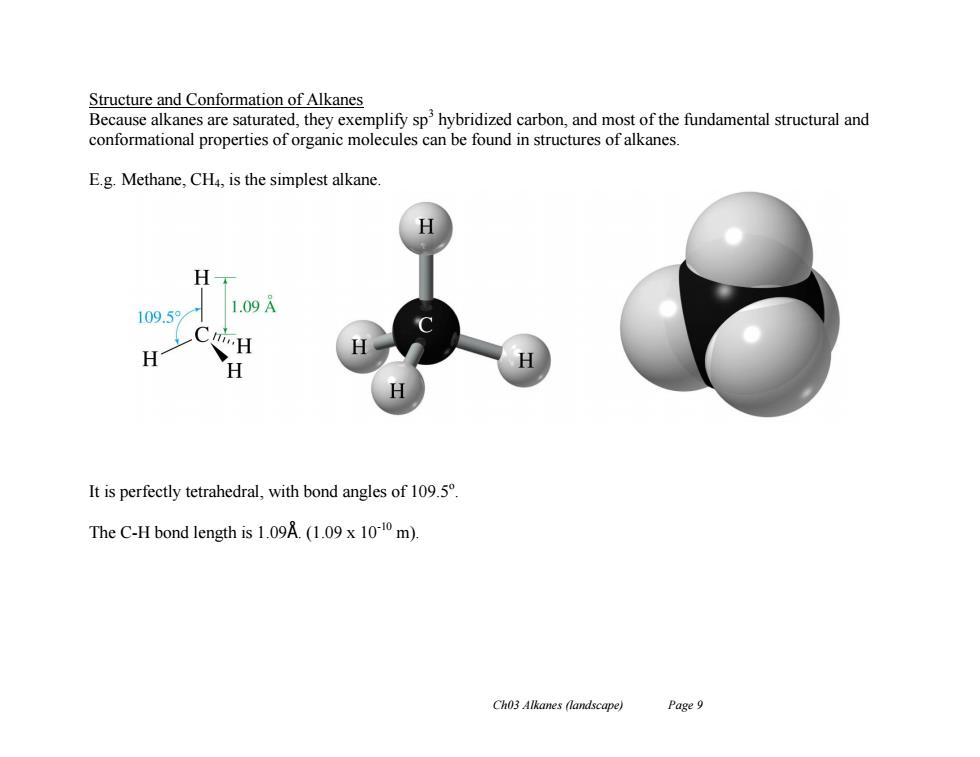
Structure and Conformation of Alkanes Because alkanes are saturated,they exemplify sphybridized carbon,and most of the fundamental structural and conformational properties of organic molecules can be found in structures of alkanes. E.g.Methane,CH4,is the simplest alkane. H 109.5 1.09A H H It is perfectly tetrahedral,with bond angles of 109.5 The C-H bond length is 1.09A.(1.09 x 1010 m). Ch03 Alkanes (landscape) Page 9
Ch03 Alkanes (landscape) Page 9 Structure and Conformation of Alkanes Because alkanes are saturated, they exemplify sp3 hybridized carbon, and most of the fundamental structural and conformational properties of organic molecules can be found in structures of alkanes. E.g. Methane, CH4, is the simplest alkane. It is perfectly tetrahedral, with bond angles of 109.5o . The C-H bond length is 1.09Å. (1.09 x 10-10 m)
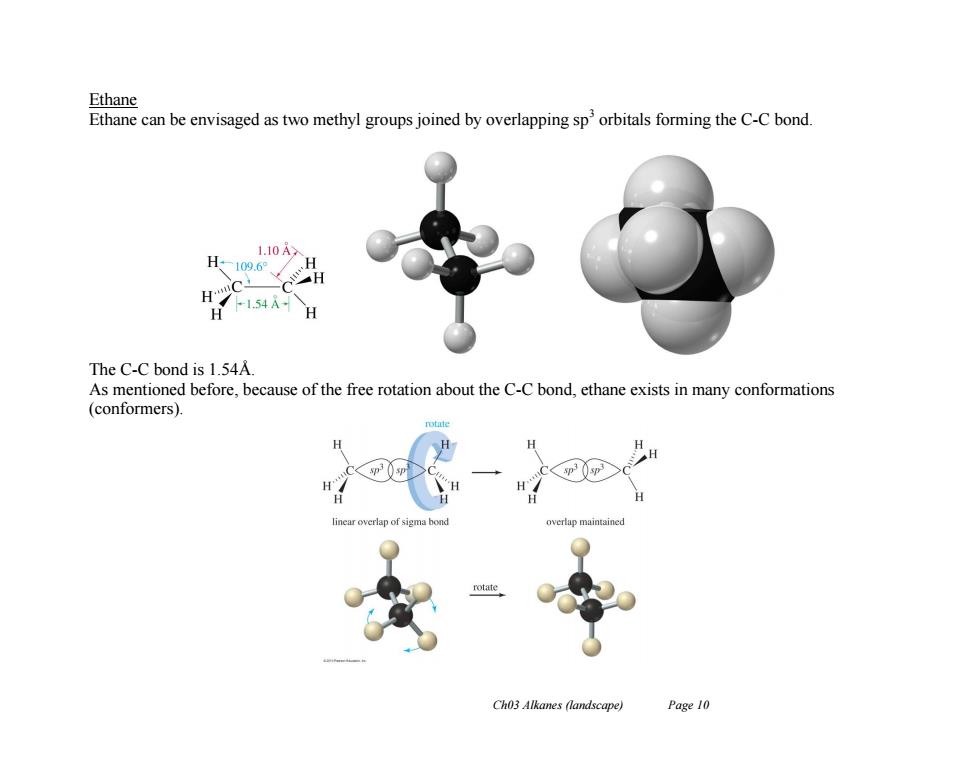
Ethane Ethane can be envisaged as two methyl groups joined by overlapping sporbitals forming the C-C bond. 1.10A H-109.6 H H 1.54A H The C-C bond is 1.54A As mentioned before,because of the free rotation about the C-C bond,ethane exists in many conformations (conformers). near overlap of sigma bon Ch03 Alkanes (landscape) Page 10
Ch03 Alkanes (landscape) Page 10 Ethane Ethane can be envisaged as two methyl groups joined by overlapping sp3 orbitals forming the C-C bond. The C-C bond is 1.54Å. As mentioned before, because of the free rotation about the C-C bond, ethane exists in many conformations (conformers)