
Non-equivalent hybridization sp3-hybrides H:N:H 出 for NH: 0=107.3° a+(1-a)c0s107.3°=0 a=0.23 L,=1-3*0.23=0.31 Other example L,=3-3*0.77=0.69 Vbonding=V0.23s+V0.77p=0.48s+0.88p PH3,PF3,NF3, Wome-m=V0.31s+V0.69p=0.56s+0.83p For d-s-p hybridization,the angles between two hybrid orbitals can be calculated by:(where a,B and y are the component ofs,p and d orbitals) a*pcoaco 3 for d'spa=1 6Bs1 31 11 13 6 2 -2-0 cos0=0,cos02=-1 Octahedral 01=90°,02=180
H N H H sp3 − hybrides X y z t 1 t 2 t 3 t 4 s p s p s p s p L L for NH lone pair bonding p s 0.31 0.69 0.56 0.83 0.23 0.77 0.48 0.88 3 3*0.77 0.69 1 3*0.23 0.31 0.23 (1 ) cos107.3 0 107.3 3 = + = + = + = + = − = = − = = + − = = ψ − ψ α α α θ o o Other example PH3, PF3, NF3, Non-equivalent hybridization For d-s-p hybridization, the angles between two hybrid orbitals can be calculated by: (where α, β and γ are the component of s, p and d orbitals) o o 90 , 180 cos 0, cos 1 ) 0 2 1 cos 2 3 ( 3 1 cos 2 1 6 1 , 3 1 , 2 1 , 6 1 for d sp , ) 0 2 1 cos 2 3 cos ( 1 2 1 2 2 2 3 2 = = = = − + + − = = = = + + − = θ θ θ θ θ θ α β γ α β θ γ θ Octahedral 5 1 3 x y z

4.The ability of the hybrid orbital fn=a+3B+5y hybrid orbital bonding ability:s,p,d,f:l,√3,√5,√7 for the sp"hybridizations, 1 0=5,B= 2 2 fn=1.932 Sp e三2Bs 3 fn=1.992 Sp2 3 0=43B= f=2.00 4 p a&=0, /6-5 6=1 a=6B-2Y-1 f=2.925dsp 5.Discussions sp2 ex.BF3(D3h),BH3(D3h),NO,CO2-, H Is atomic orbital Example:BH3 B sp2 hybrid orbital .3 sp2 hybrid orbitals each with one electron. .This one electron pairs with the hydrogen electron
= α + 3β + 5γ hf 1, 1 0, 3 2.00 sp 4 3 , 4 1 1.992 sp 3 2 , 3 1 1.932 sp 2 1 , 2 1 for the sp hybridizations, 3 2 n = = = = = = = = = = = = = h h h h h f f f f f α α α β α β α β 4. The ability of the hybrid orbital hybrid orbital bonding ability :s, p, d, f :1, 3, 5, 7 2 3 2.925 d sp 3 1 , 2 1 , 6 1 α = β = γ = fh = Example: BH3 ex. BF3(D3h), BH3 (D3h), NO3 - , CO3 2-, sp2 B F F F •3 sp2 hybrid orbitals each with one electron. •This one electron pairs with the hydrogen electron. 5. Discussions

Example: H t红3 H C2H4 C2H4 Bend bonding cyclo-triethyle H2 H2C CH2 Sp3
tr1 tr2 x y tr3 C2H4 Example: C H H C H H C2H4 π pz-pz Bend bonding cyclo-triethyle H2C CH2 H2 C sp3

5.2 Valence Shell Electron-Pair Repulsion (VSEPR)Model Quantum mechanical treatments have a number of advantages.However,the VSEPR model allows a simple qualitative prediction of molecular geometry. VSEPR Atom B in Ab lie on the surface of a sphere;electron pairs are "localized"on a sphere of smaller radius at maximum distances apart,so as to minimize overlap of different electron pairs. 1.Repulsion between two electron pairs is the greatest 2.Repulsion between between a lone electron pair and a bonding electron pair is less. 3.Repulsion between two bonding pairs is the least. 4.electron pair do not influence stereochemistry
§5.2 Valence Shell Electron-Pair Repulsion (VSEPR) Model • Quantum mechanical treatments have a number of advantages. However, the VSEPR model allows a simple qualitative prediction of molecular geometry. VSEPR Atom B in Abn lie on the surface of a sphere; electron pairs are “localized” on a sphere of smaller radius at maximum distances apart, so as to minimize overlap of different electron pairs. 1. Repulsion between two electron pairs is the greatest. 2. Repulsion between between a lone electron pair and a bonding electron pair is less. 3. Repulsion between two bonding pairs is the least. 4. π electron pair do not influence stereochemistry
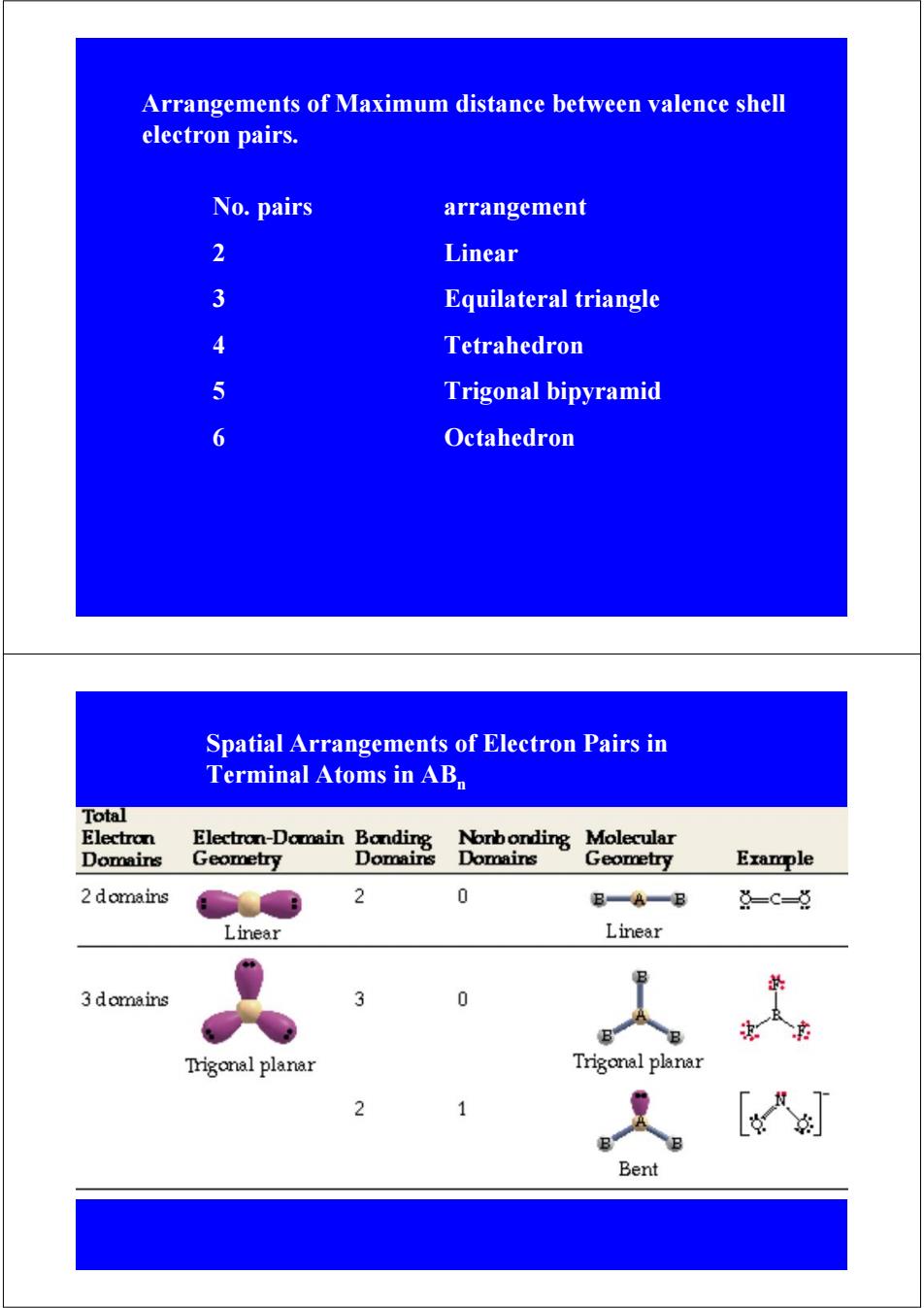
Arrangements of Maximum distance between valence shell electron pairs. No.pairs arrangement 2 Linear 3 Equilateral triangle 4 Tetrahedron 5 Trigonal bipyramid 6 Octahedron Spatial Arrangements of Electron Pairs in Terminal Atoms in AB Total Electron Electron-Domain Bonding Norbonding Molecular Domains Geometry Domains Domains Geometry Example 2 domains 2 0 B一A一B 点-C=草 Linear Linear 3 domains 3 0 Trigonal planar Trigonal planar [入 Bent
Arrangements of Maximum distance between valence shell electron pairs. No. pairs arrangement 2 Linear 3 Equilateral triangle 4 Tetrahedron 5 Trigonal bipyramid 6 Octahedron Spatial Arrangements of Electron Pairs in Terminal Atoms in ABn