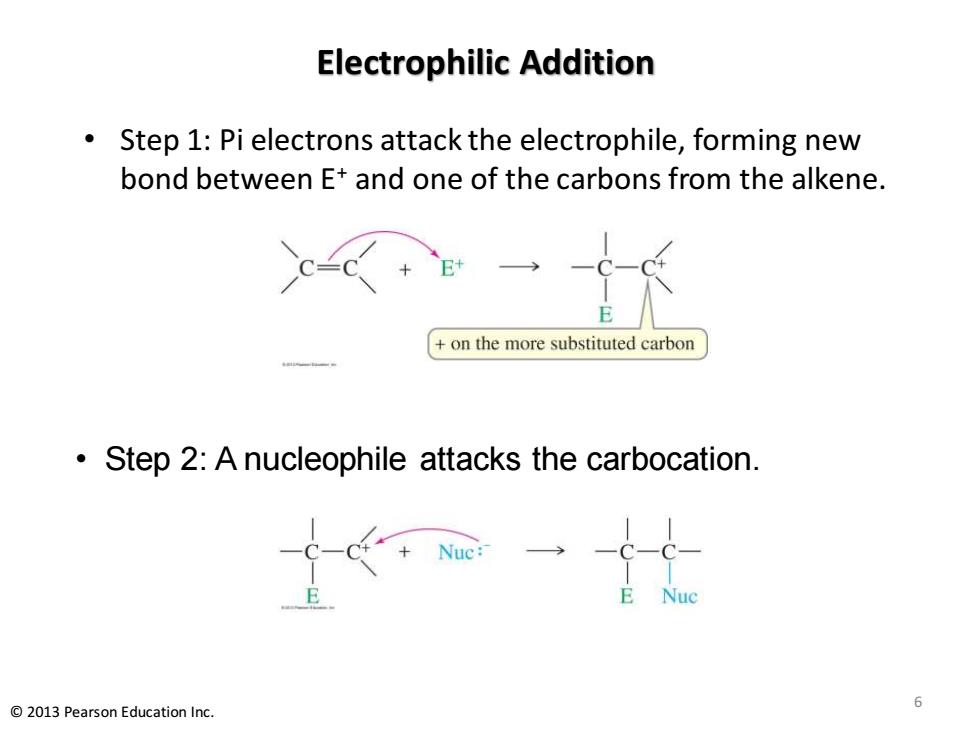
Electrophilic Addition Step 1:Pi electrons attack the electrophile,forming new bond between E+and one of the carbons from the alkene. on the more substituted carbon Step 2:A nucleophile attacks the carbocation. 6 2013 Pearson Education Inc
Electrophilic Addition • Step 1: Pi electrons attack the electrophile, forming new bond between E+ and one of the carbons from the alkene. • Step 2: A nucleophile attacks the carbocation. 6 © 2013 Pearson Education Inc
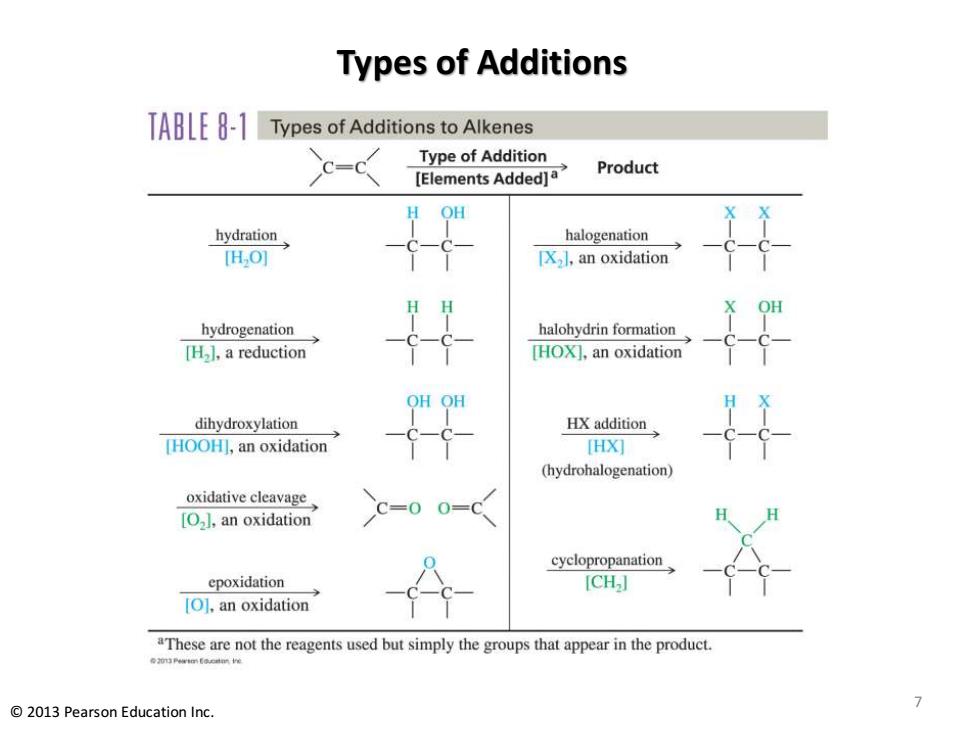
Types of Additions TABLE8-1 Types of Additions to Alkenes c=c Type of Addition [Elements Added]a Product OH hydration halogenation [H,O] IX.I,an oxidation hydrogenation halohydrin formation [H].a reduction [HOX],an oxidation 平 dihydroxylation HX addition [HOOH],an oxidation HX灯 4 (hydrohalogenation) oxidative cleavage C=0 [O].an oxidation 0=0 cyclopropanation epoxidation [CH] [O].an oxidation "These are not the reagents used but simply the groups that appear in the product. 0口ertm Educatm Ie 7 2013 Pearson Education Inc
Types of Additions 7 © 2013 Pearson Education Inc
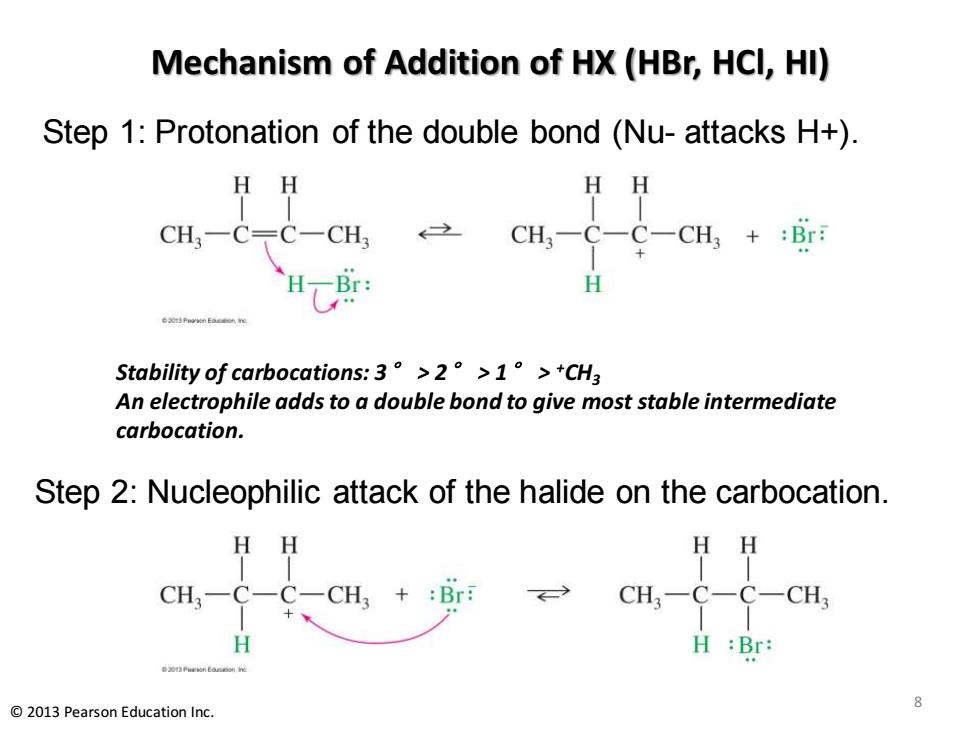
Mechanism of Addition of HX(HBr,HCl,HI) Step 1:Protonation of the double bond (Nu-attacks H+) HH HH CH3一C〒C-CH CH3-C-C-CH3+:Br: H Stability of carbocations:.3°>2°>1。>+CH3 An electrophile adds to a double bond to give most stable intermediate carbocation. Step 2:Nucleophilic attack of the halide on the carbocation. HH HH CH3一C-C一CH3+:Br CH3-C-C- CH H H :Br: 2013 Pearson Education Inc
Mechanism of Addition of HX (HBr, HCl, HI) Step 1: Protonation of the double bond (Nu- attacks H+). Step 2: Nucleophilic attack of the halide on the carbocation. 8 © 2013 Pearson Education Inc. Stability of carbocations: 3° > 2° > 1° > +CH3 An electrophile adds to a double bond to give most stable intermediate carbocation
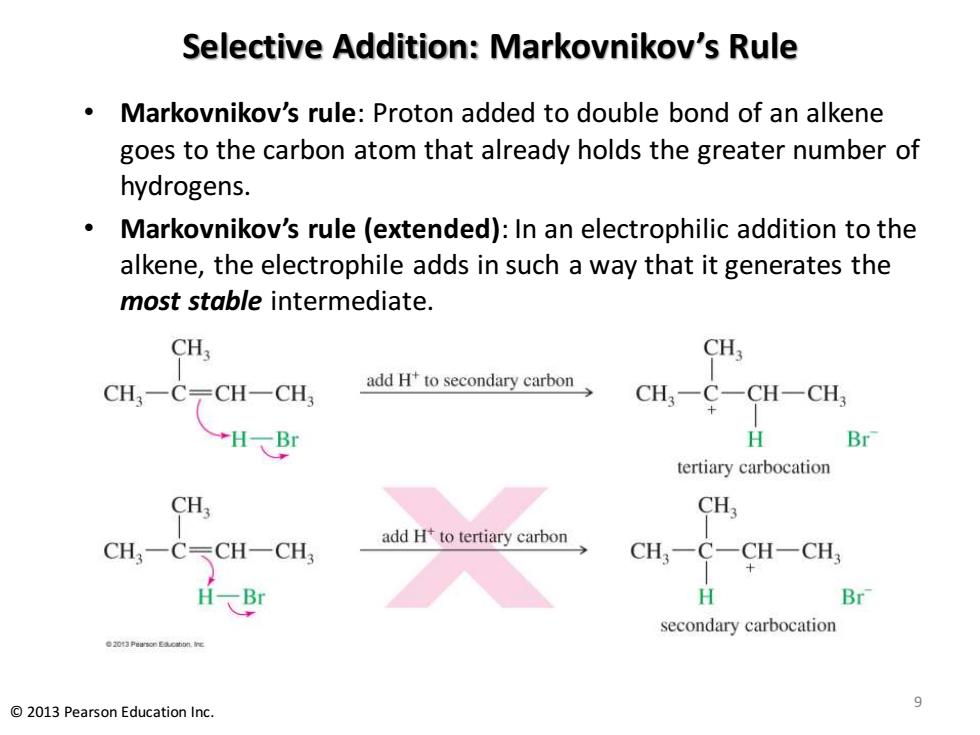
Selective Addition:Markovnikov's Rule 。 Markovnikov's rule:Proton added to double bond of an alkene goes to the carbon atom that already holds the greater number of hydrogens. Markovnikov's rule (extended):In an electrophilic addition to the alkene,the electrophile adds in such a way that it generates the most stable intermediate. CHs CHs CH3-C-CH-CH3 add Hto secondary carbon CH,-C-CH-CH, →HBr H Br tertiary carbocation CH3 CH一CCH-CH add H to tertiary carbon CH,-C-CH-CH; H Br secondary carbocation 9 2013 Pearson Education Inc
Selective Addition: Markovnikov’s Rule • Markovnikov’s rule: Proton added to double bond of an alkene goes to the carbon atom that already holds the greater number of hydrogens. • Markovnikov’s rule (extended): In an electrophilic addition to the alkene, the electrophile adds in such a way that it generates the most stable intermediate. 9 © 2013 Pearson Education Inc
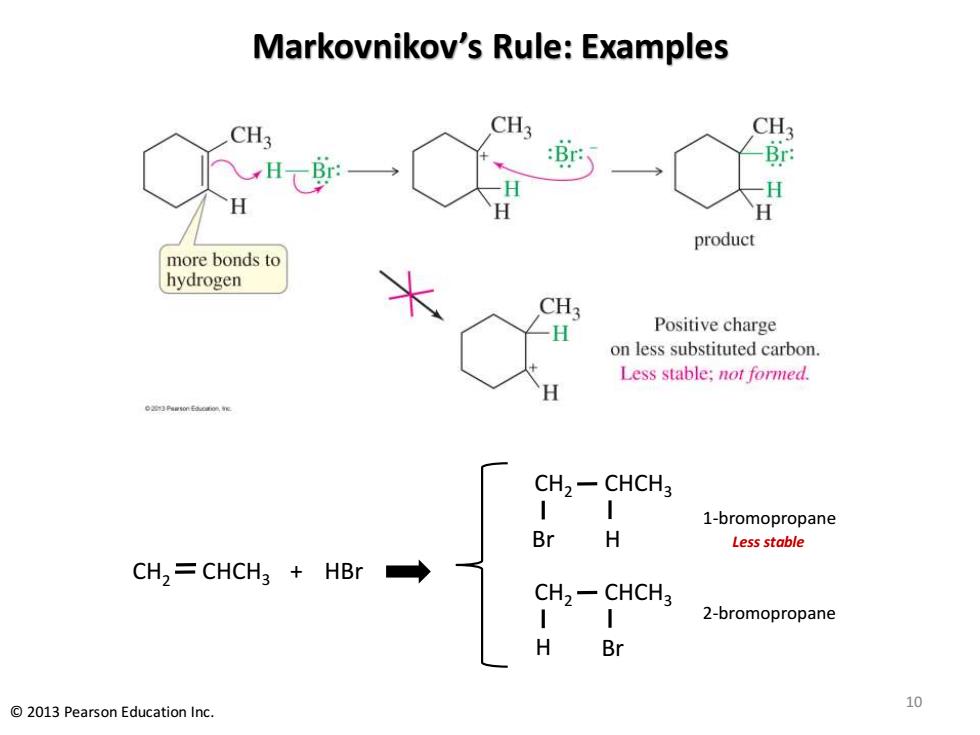
Markovnikov's Rule:Examples CH3 CH3 CH3 : H H product more bonds to hydrogen CH3 H Positive charge on less substituted carbon. Less stable:not formed. CH2-CHCH3 1-bromopropane Br H Less stable CH,=CHCH3 HBr CH2-CHCH3 2-bromopropane H Br 10 2013 Pearson Education Inc
Markovnikov’s Rule: Examples 10 © 2013 Pearson Education Inc. CH2 CHCH3 Br + H HBr CH2 CHCH3 H Br CH2 CHCH3 1-bromopropane 2-bromopropane Less stable