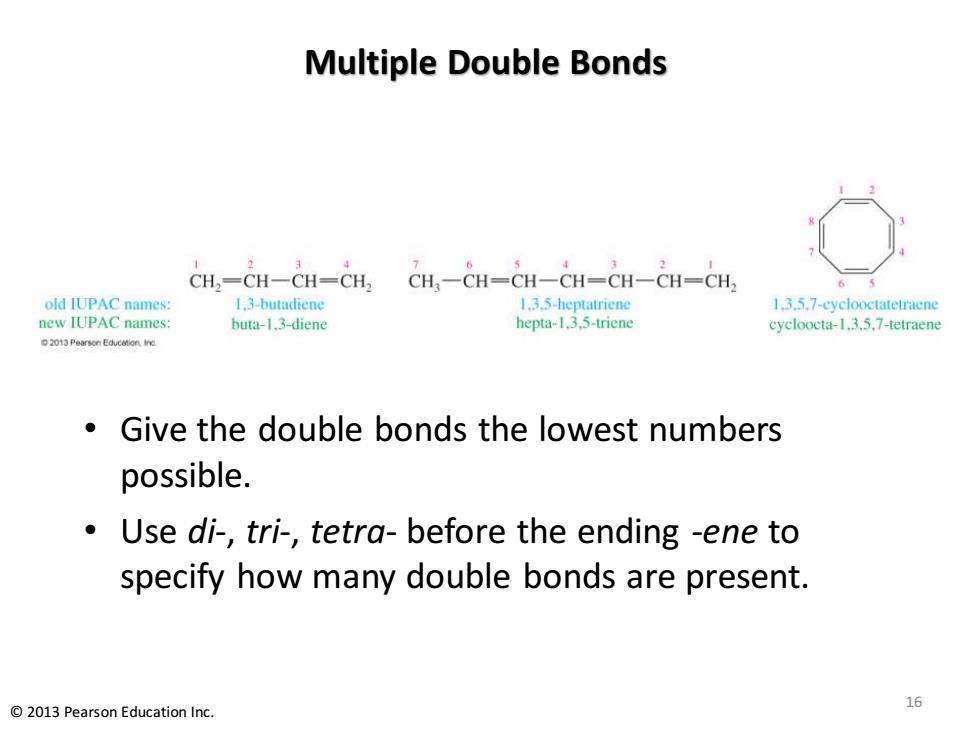
Multiple Double Bonds CH,-CH-CH-CH, 2 CH,-CH-CH-CH-CH-CH-CH; old IUPAC names: 1.3-butadiene 1.3.5-heptatriene 1.3.5.7-cyclooctatetraene new IUPAC names: buta-1.3-diene hepta-1.3.5-triene cycloocta-1.3.5.7-tetraene 2013 Pearson Education Ine Give the double bonds the lowest numbers possible. Use di-,tri-,tetra-before the ending -ene to specify how many double bonds are present. 16 2013 Pearson Education Inc
Multiple Double Bonds • Give the double bonds the lowest numbers possible. • Use di-, tri-, tetra- before the ending -ene to specify how many double bonds are present. 16 © 2013 Pearson Education Inc
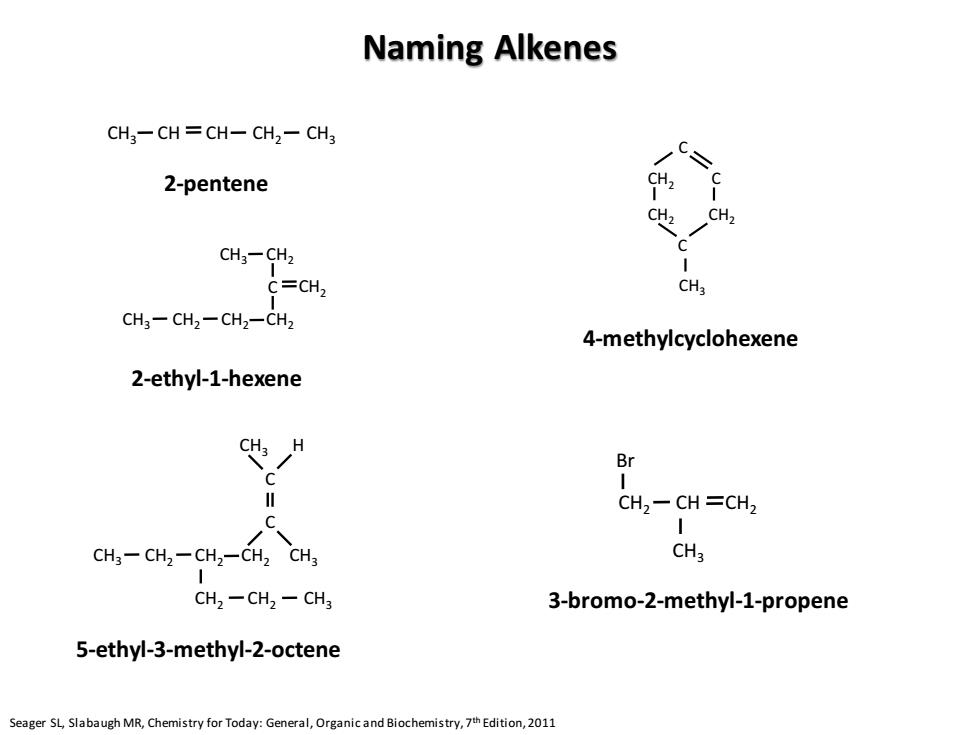
Naming Alkenes CH3-CH CH-CH2-CH3 C= 2-pentene CH2 CH2 CH3-CH2 C C=CH2 CH3 CH3-CH2-CH2-CH2 4-methylcyclohexene 2-ethyl-1-hexene CH3 H Br CH2-CH=CH2 C CH3-CH2-CH2-CH2 CH3 CHa CH2-CH2-CH3 3-bromo-2-methyl-1-propene 5-ethyl-3-methyl-2-octene Seager SL,Slabaugh MR,Chemistry for Today:General,Organic and Biochemistry,7th Edition,2011
Naming Alkenes Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7th Edition, 2011 CH3 CH CH CH2 CH3 CH3 CH2 C CH2 CH3 CH2 CH2 CH2 C CH3 CH2 CH2 CH2 CH2 CH2 CH3 CH3 C CH3 H 2-pentene 2-ethyl-1-hexene 5-ethyl-3-methyl-2-octene C CH2 CH2 CH2 C C CH3 CH2 CH2 CH3 Br CH 4-methylcyclohexene 3-bromo-2-methyl-1-propene

Cis-Trans Isomers H.C CH2CH H.C H.C H H CH2CH2CH: H CH2CH; HC CH2CH: cis-2-pentene frans-2-pentene 2-methyl-2-pentene I-pentene cis-pent-2-ene trans-pent-2-ene 2-methylpent-2-ene pent-1-ene (neither cis nor trans) Also called geometric isomerism. Similar groups on same side of double bond,alkene is cis. Similar groups on opposite sides of double bond,alkene is trans. Not all alkenes show cis-trans isomerism. 2013 Pearson Education Inc. 18
Cis-Trans Isomers • Also called geometric isomerism. • Similar groups on same side of double bond, alkene is cis. • Similar groups on opposite sides of double bond, alkene is trans. • Not all alkenes show cis-trans isomerism. 18 © 2013 Pearson Education Inc