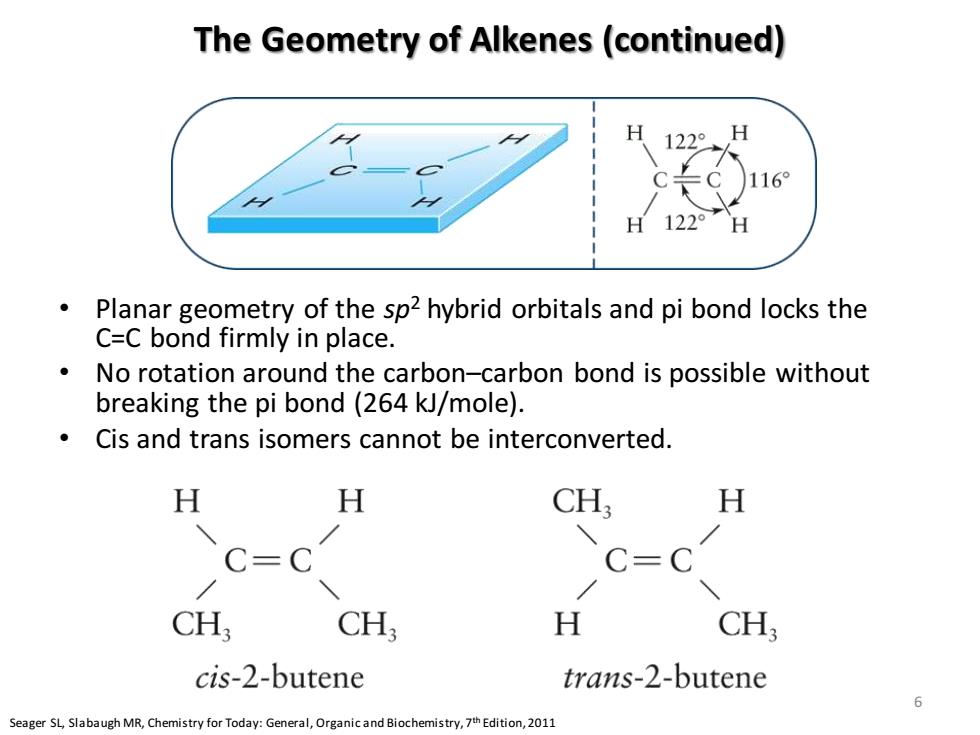
The Geometry of Alkenes(continued) 16 H 122° Planar geometry of the sp2 hybrid orbitals and pi bond locks the C=C bond firmly in place. No rotation around the carbon-carbon bond is possible without breaking the pi bond(264 kJ/mole). Cis and trans isomers cannot be interconverted. H H CH H C=C C=C CH; CH H CH, cis-2-butene trans-2-butene 6 Seager SLSlabaugh MR,Chemistry for Today:General,Organicand Biochemistry,Edition,2011
The Geometry of Alkenes (continued) • Planar geometry of the sp2 hybrid orbitals and pi bond locks the C=C bond firmly in place. • No rotation around the carbon–carbon bond is possible without breaking the pi bond (264 kJ/mole). • Cis and trans isomers cannot be interconverted. Seager SL, Slabaugh MR, Chemistry for Today: General, Organic and Biochemistry, 7th Edition, 2011 6
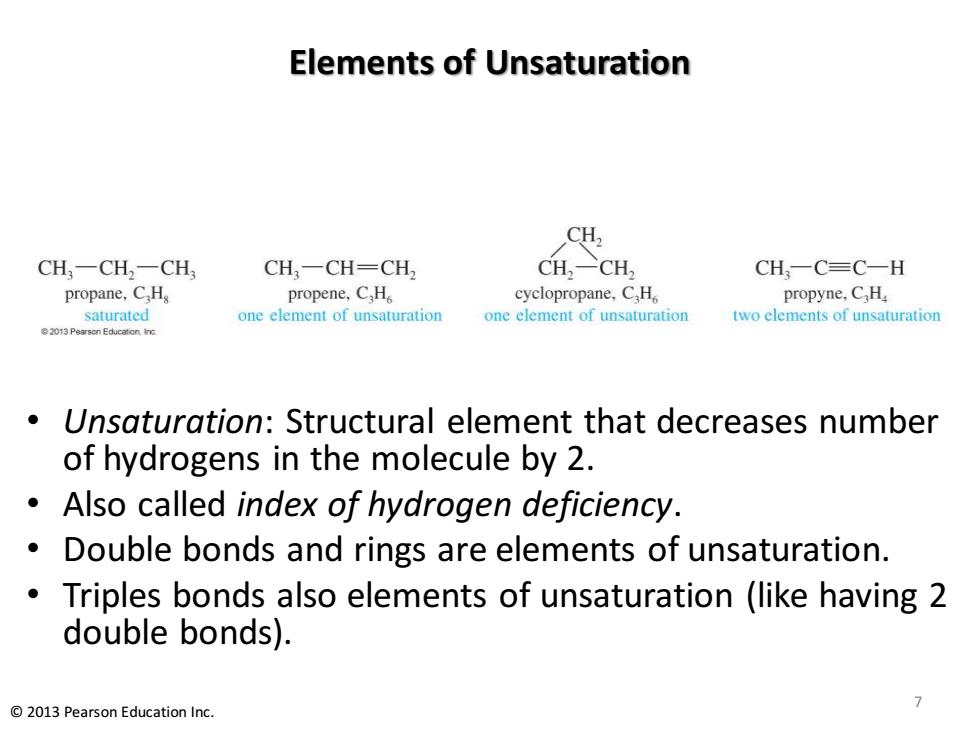
Elements of Unsaturation CH, CH,一CH2一CH CH3一CH=CH CH2-CH, CH,一C=C-H propane,C.H propene,C,H。 cyclopropane,C,H propyne,C.H saturated one element of unsaturation one element of unsaturation two elements of unsaturation 2013 Pearson Education.inc Unsaturation:Structural element that decreases number of hydrogens in the molecule by 2. Also called index of hydrogen deficiency. Double bonds and rings are elements of unsaturation. Triples bonds also elements of unsaturation (like having 2 double bonds). 2013 Pearson Education Inc
Elements of Unsaturation • Unsaturation: Structural element that decreases number of hydrogens in the molecule by 2. • Also called index of hydrogen deficiency. • Double bonds and rings are elements of unsaturation. • Triples bonds also elements of unsaturation (like having 2 double bonds). 7 © 2013 Pearson Education Inc
Calculating Unsaturation To calculate number of unsaturations: Find the number of hydrogens the carbons would have if the compounds were saturated(CnH2n+2) Subtract the actual number of hydrogens and divide by 2. This calculation cannot distinguish between unsaturations from multiple bonds and those from rings. 2013 Pearson Education Inc
Calculating Unsaturation To calculate number of unsaturations: • Find the number of hydrogens the carbons would have if the compounds were saturated (CnH2n+2). • Subtract the actual number of hydrogens and divide by 2. • This calculation cannot distinguish between unsaturations from multiple bonds and those from rings. 8 © 2013 Pearson Education Inc
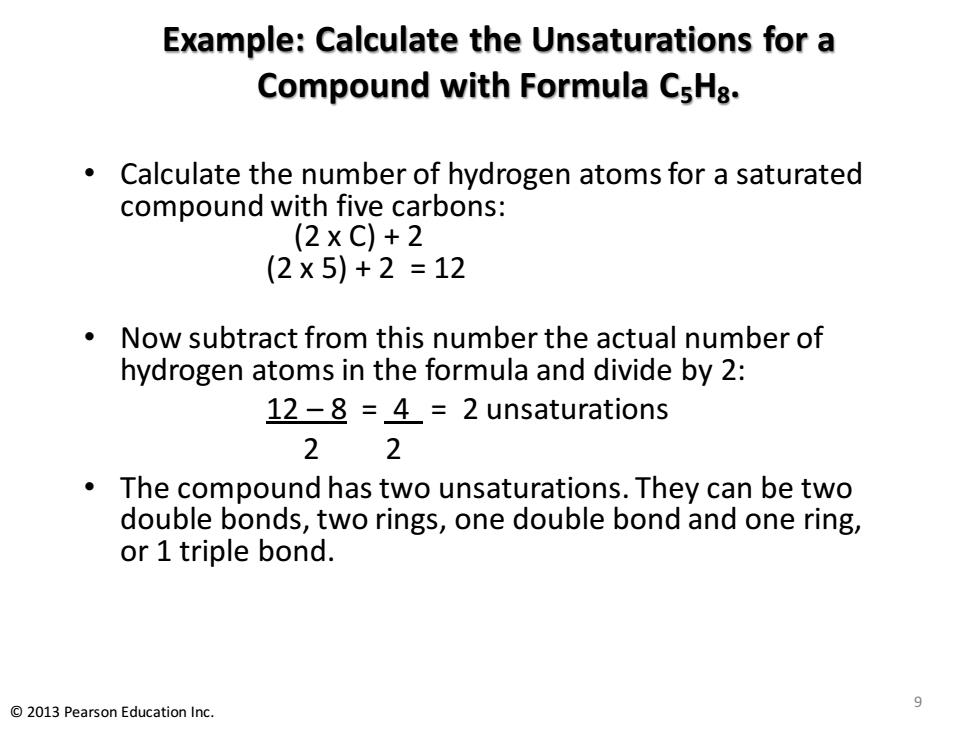
Example:Calculate the Unsaturations for a Compound with Formula CsHa. Calculate the number of hydrogen atoms for a saturated compound with five carbons: (2XC)+2 (2×5)+2=12 Now subtract from this number the actual number of hydrogen atoms in the formula and divide by 2: 12-8 =4=2 unsaturations 22 The compound has two unsaturations.They can be two double bonds,two rings,one double bond and one ring, or 1 triple bond. 9 2013 Pearson Education Inc
Example: Calculate the Unsaturations for a Compound with Formula C5H8. • Calculate the number of hydrogen atoms for a saturated compound with five carbons: (2 x C) + 2 (2 x 5) + 2 = 12 • Now subtract from this number the actual number of hydrogen atoms in the formula and divide by 2: 12 – 8 = 4 = 2 unsaturations 2 2 • The compound has two unsaturations. They can be two double bonds, two rings, one double bond and one ring, or 1 triple bond. 9 © 2013 Pearson Education Inc
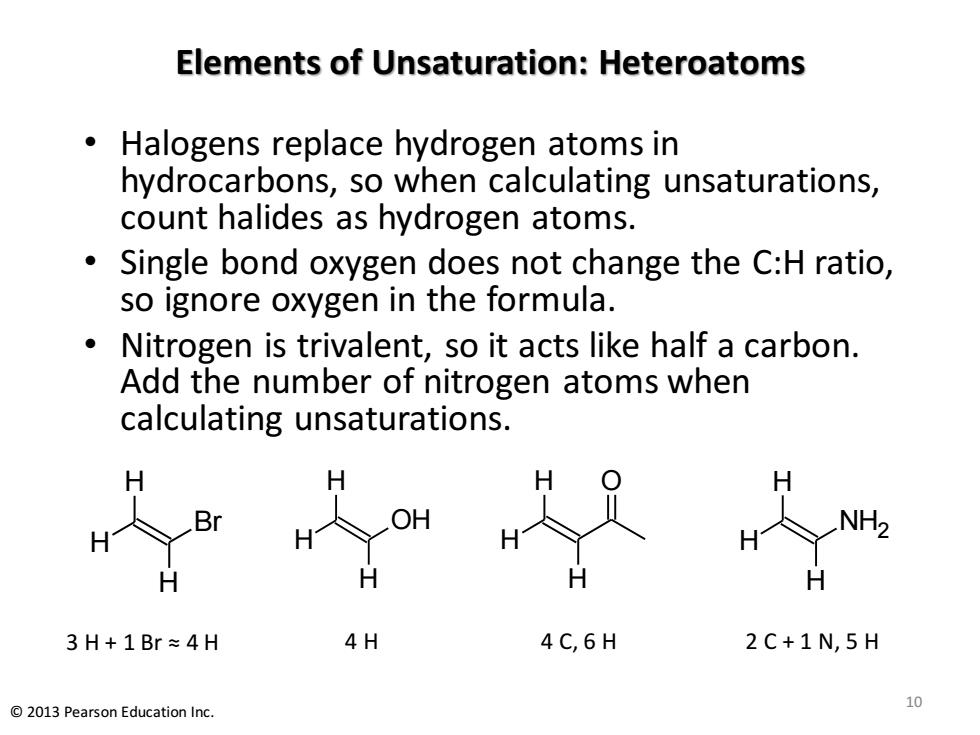
Elements of Unsaturation:Heteroatoms Halogens replace hydrogen atoms in hydrocarbons,so when calculating unsaturations, count halides as hydrogen atoms. Single bond oxygen does not change the C:H ratio, so ignore oxygen in the formula. Nitrogen is trivalent,so it acts like half a carbon. Add the number of nitrogen atoms when calculating unsaturations. H OH NH2 3H+1Br≈4H 4H 4C,6H 2C+1N,5H 2013 Pearson Education Inc. 0
Elements of Unsaturation: Heteroatoms • Halogens replace hydrogen atoms in hydrocarbons, so when calculating unsaturations, count halides as hydrogen atoms. • Single bond oxygen does not change the C:H ratio, so ignore oxygen in the formula. • Nitrogen is trivalent, so it acts like half a carbon. Add the number of nitrogen atoms when calculating unsaturations. 10 © 2013 Pearson Education Inc. H H H Br 3 H + 1 Br ≈ 4 H H H H OH 4 H H O H H 4 C, 6 H H H H NH2 2 C + 1 N, 5 H