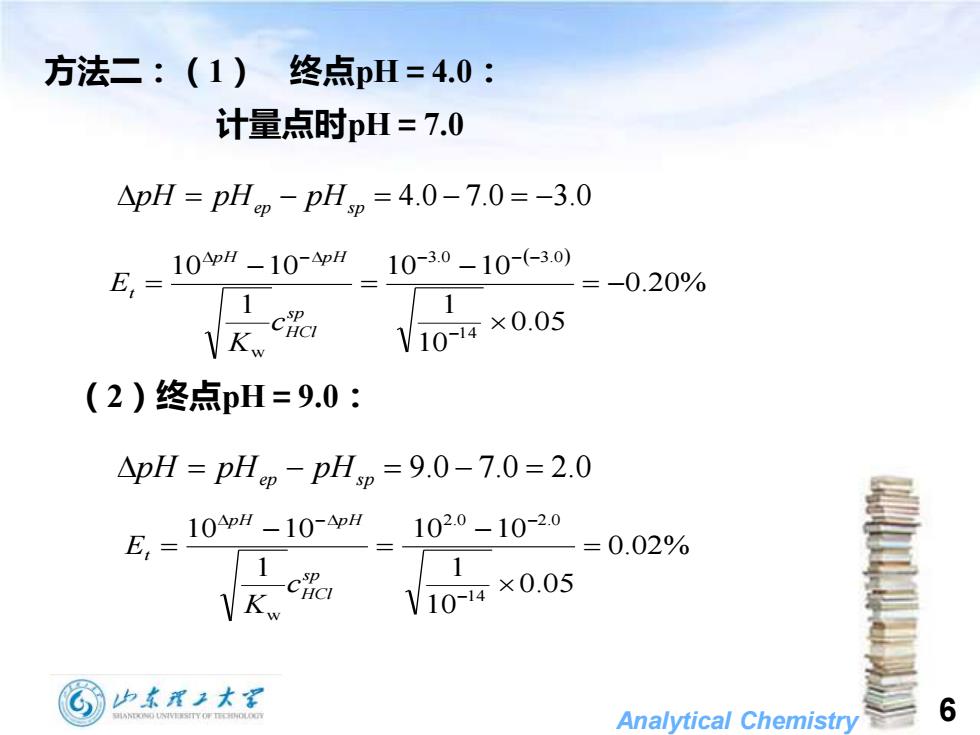
方法二:(1)终点pH=4.0: 计量点时pH=7.0 △pH=pHm-pHm=4.0-7.0=-3.0 10pH-10-pH10-3.0-10-(-3.0) =-0.20% V10-14×0.05 (2)终点pH=9.0: pH=pHp-pHp=9.0-7.0=2.0 E,=100-104p 102.0-10-2.0 =0.02% SP 1 -CHCI V10-14×0.05 山东理王大 Analytical Chemistry 6
Analytical Chemistry 6 方法二:(1) 终点pH=4.0: 计量点时pH=7.0 pH = pHep − pHs p = 4.0 − 7.0 = −3.0 ( ) 0.20% 0.05 10 1 10 10 1 10 10 1 4 3.0 3.0 w = − − = − = − − − − − s p HCl p H p H t c K E (2)终点pH=9.0: pH = pHep − pHs p = 9.0 − 7.0 = 2.0 0.02% 0.05 10 1 10 10 1 10 10 1 4 2.0 2.0 w = − = − = − − − s p HCl p H p H t c K E
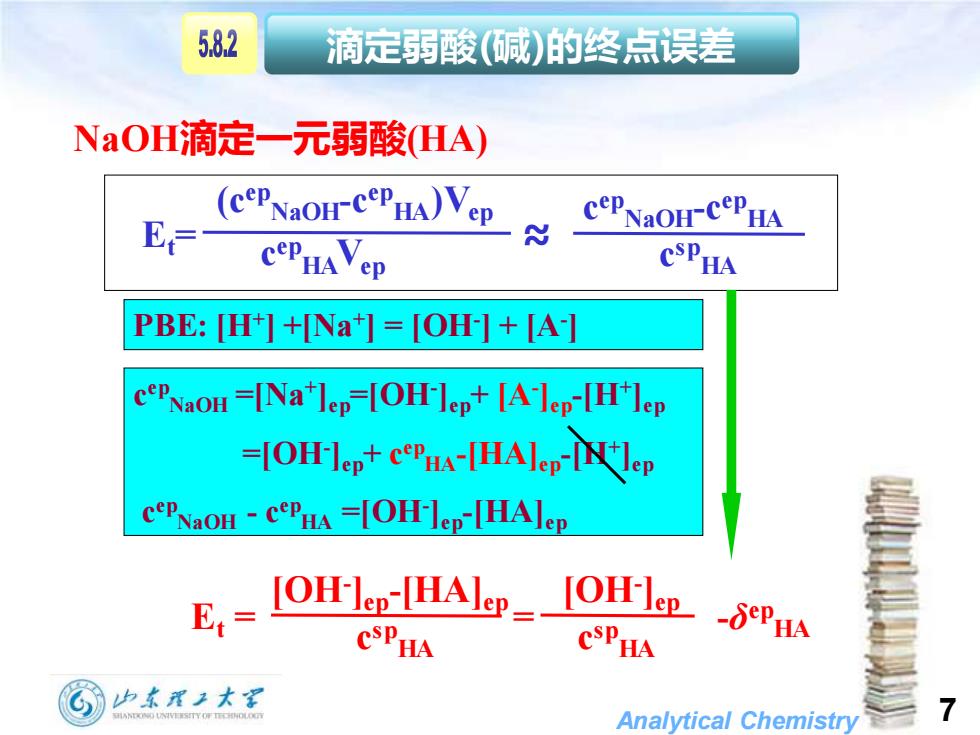
5.82 滴定弱酸(碱)的终点误差 NaOH滴定一元弱酸HA) (cePNaoH-CePHA)Vep E N cePNaoH-CePHA COPHAVep CSPHA PBE:[H+]+[Na+]=[OH-]+[A-] cePNaoH=[Nalep=[OH-lep+[Alep-[H]ep -OH-lep+cPHA-THAlep-IN lep cePNaon -cPHA =[OH-]ep-[HAlep E IOHl-HAP CSPHA CSPHA 山东理王大军 Analytical Chemistry
Analytical Chemistry 7 NaOH滴定一元弱酸(HA) 滴定弱酸(碱)的终点误差 Et= ≈ (cep NaOH-c ep HA)Vep c ep HAVep c ep NaOH-c ep HA c sp HA PBE: [H+ ] +[Na+ ] = [OH- ] + [A- ] c ep NaOH =[Na+ ]ep=[OH- ]ep+ [A- ]ep-[H+ ]ep =[OH- ]ep+ c ep HA-[HA]ep-[H+ ]ep c ep NaOH - c ep HA =[OH- ]ep-[HA]ep Et = = -δ ep HA [OH- ]ep-[HA]ep c sp HA [OH- ]ep c sp HA
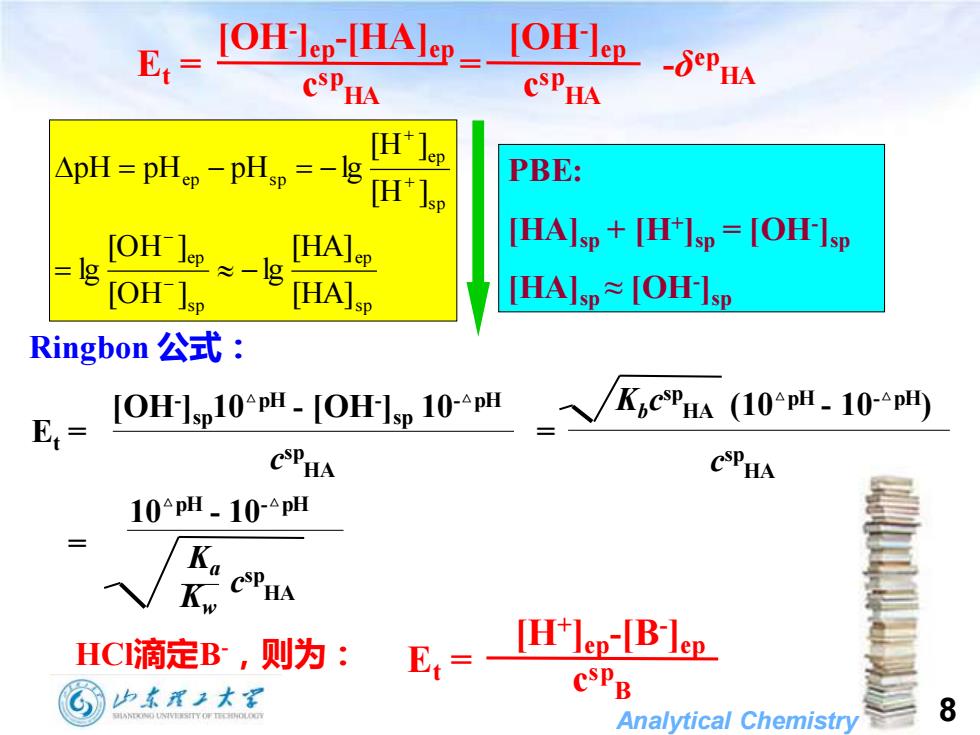
E,=OlealHlm=Olampn CSPHA CSPHA [H'lep ApH =pH PBE: [OH lep [HA]ep [HAIsp+[H+Isp=[OH-Isp [OH -lg [HA]sp HAsp≈[OH-Isp Ringbon公式: E= [OH-]sp10PH-[OH-ISp 10-PH KiCSPHA (10PH-10-PH) CSPHA CSPHA 10pH.10-pH Ka HCI滴定B,则为: E= [H'lep-[B-lep 也本眉2大里 Analytical Chemistry 8
Analytical Chemistry 8 Ringbon 公式: HCl滴定B-,则为: Et = [H+ ]ep-[B- ]ep c sp B Et = = -δ ep HA [OH- ]ep-[HA]ep c sp HA [OH- ]ep c sp HA s p ep s p ep s p ep ep s p [HA] [HA] lg [OH ] [OH ] lg [H ] [H ] pH pH pH lg = − = − = − − − + + Et = = c sp HA [OH- ]sp10△pH - [OH- ]sp 10-△pH Kbc sp HA c sp HA (10△pH - 10-△pH) PBE: [HA]sp + [H+ ]sp = [OH- ]sp [HA]sp ≈ [OH- ]sp Kw Ka c sp HA 10△pH - 10-△pH =

例1用0.1200mol/L的Na0H滴定30.00mL0.1000mol/L的HAc, 求滴至pH为7.0和9.0时的滴定误差。 [解]:计量点时消耗的NaOH体积为: A=25.00mL e 0.1000×30.00=0.054moL CNaOH 25.00+30.00 [OH1p=-Vc.K,=10526 pHsp-8.74 (1)若pHep7.0时 △pH=-1.74 E=VK,(10p1.10pn) V1092510174-1037)-0.55% Vc曜 √0.054 (2)若pHp=9.0时△p=0.26 E,=1092(106-1002 =0.01% V0.054 山东理子大军 Analytical Chemistry 9
Analytical Chemistry 9 [解]:计量点时消耗的NaOH 体积为: pHsp=8.74 5.26 sp b [OH ] c K 10 − − = = (1)若pHep=7.0时 △pH=-1.74 25.00mL NaOH HAc NaOH = = c cV V 0.054mol/L 25.00 30.00 0.1000 30.00 HAc s p NaAc s p = + c = c = 例1 用0.1200mol/L的NaOH滴定30.00mL 0.1000mol/L的HAc , 求滴至pH为7.0和9.0时的滴定误差。 0.55% 0.054 10 (10 -10 ) c (10 -10 ) 9.2 6 1.7 4 1.74 s p HA p H - p H b t = = = − − − K E (2)若pHep=9.0时 △pH=0.26 0.01% 0.054 10 (10 -10 ) E 9.2 6 0.2 6 0.2 6 = = − − t
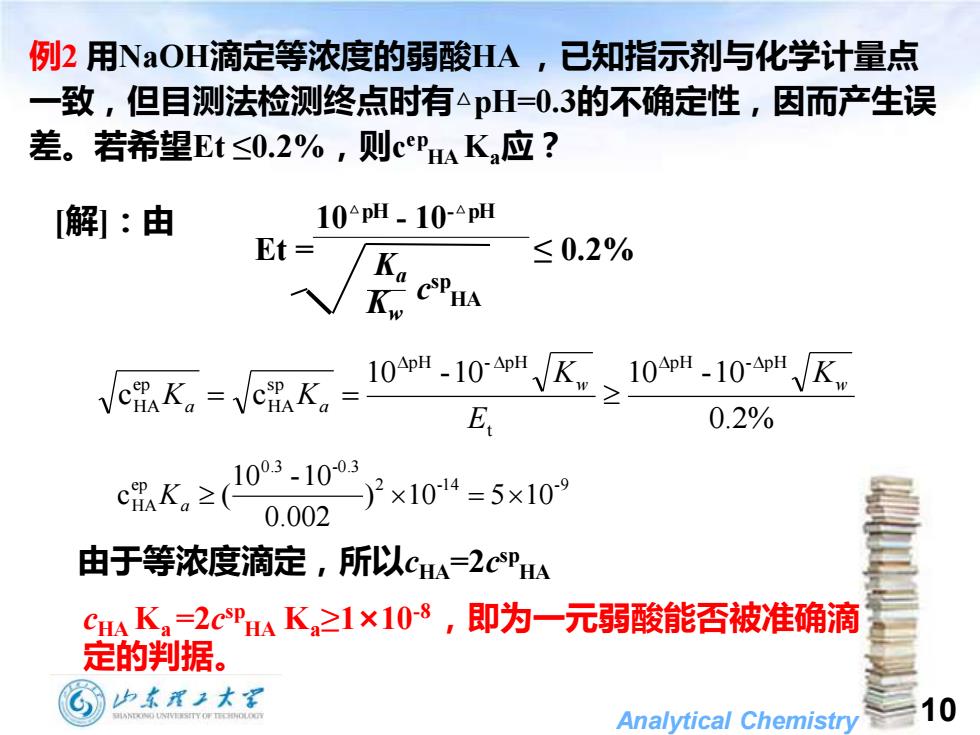
例2用NaOH滴定等浓度的弱酸HA,已知指示剂与化学计量点 一致,但目测法检测终点时有△pH=0.3的不确定性,因而产生误 差。若希望Et≤0.2%,则CPHA K应? [解]:由 10pH.10-△pH Et= Ka ≤0.2% 品K.=e品K,-10-10K≥10*-10区 E 0.2% c0K,≥103-1003 0.002 )2×1014=5x10 由于等浓度滴定,所以cA=2cPHA C4Ka=2 CSPHA K之1×10-8,即为一元弱酸能否被准确滴 定的判据。 G也本眉2大学 Analytical Chemistry 10
Analytical Chemistry 10 [解]:由 例2 用NaOH滴定等浓度的弱酸HA ,已知指示剂与化学计量点 一致,但目测法检测终点时有△pH=0.3的不确定性,因而产生误 差。若希望Et ≤0.2%,则c ep HA Ka应? Kw Ka c sp HA 10△pH - 10-△pH Et = ≤ 0.2% 2 -1 4 -9 0.3 -0.3 ep HA ) 10 5 10 0.002 10 -10 c Ka ( = 由于等浓度滴定,所以cHA=2c sp HA cHA Ka=2c sp HA Ka≥1×10-8 ,即为一元弱酸能否被准确滴 定的判据。 0.2% 10 -10 10 -10 c c p H - p H t p H - p H s p HA ep HA w w a a K E K K K = =