
Energy Diagram of Enolate Reaction enolate ion reacts with E+ R一C-CH一R +H20 R-C-CH2-R' +OH R-C-CH-R' E Copyright2010 Pearson Prentice Hall,Inc Even though the keto-enol tautomerism equilibrium favors the keto form,addition of an electrophile shifts the equilibrium toward the formation of more enol. Chapter 22 11
Chapter 22 11 Energy Diagram of Enolate Reaction ▪ Even though the keto–enol tautomerism equilibrium favors the keto form, addition of an electrophile shifts the equilibrium toward the formation of more enol
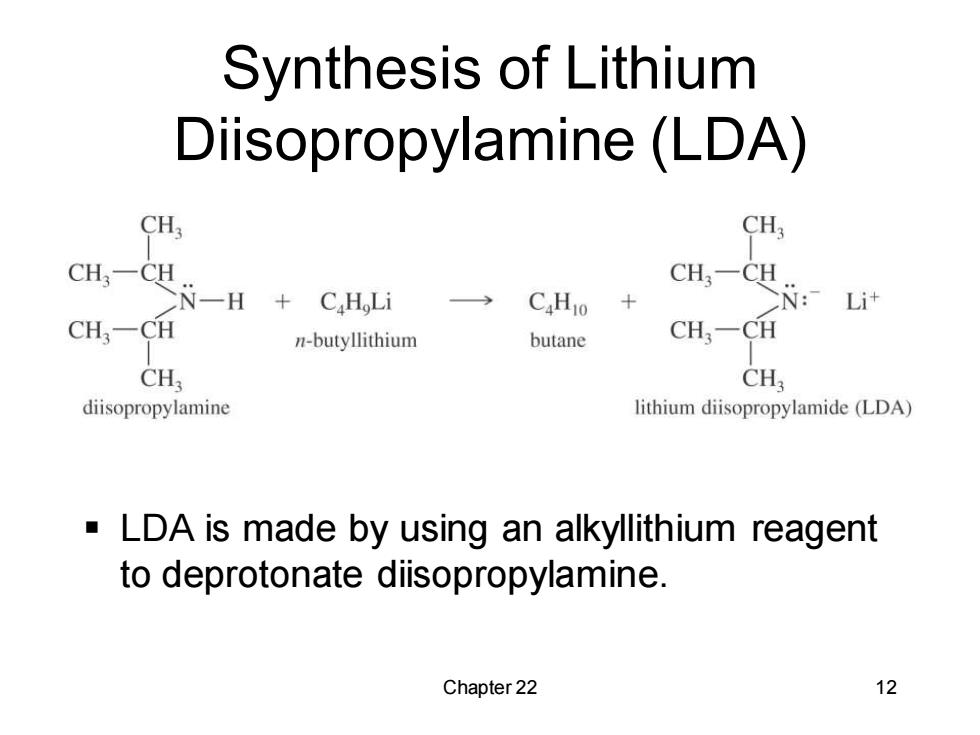
Synthesis of Lithium Diisopropylamine(LDA) CH3 CH3 CH一CH N-H C.H Li CaHo CH-c Li计 CH:-CH n-butyllithium butane CH;-CH CH CH; diisopropylamine lithium diisopropylamide(LDA) LDA is made by using an alkyllithium reagent to deprotonate diisopropylamine. Chapter 22 12
Chapter 22 12 Synthesis of Lithium Diisopropylamine (LDA) ▪ LDA is made by using an alkyllithium reagent to deprotonate diisopropylamine

Enolate of Cyclohexanone Example (i-CH)N-Lit (i-C,H)2N一H cyclohexanone LDA lithium enolate (pK.=36) (pK=19) of cyclohexanone (100%) When LDA reacts with a ketone,it abstracts the a-proton to form the lithium salt of the enolate. Chapter 22 13
Chapter 22 13 Enolate of Cyclohexanone ▪ When LDA reacts with a ketone, it abstracts the a-proton to form the lithium salt of the enolate

The a Halogenation of Ketones H -C-C- +-OH X, +X+H,0 ketone (X2 =Cl2,Br2,or I2) a-haloketone When a ketone is treated with a halogen and a base, an a halogenation reaction occurs. The reaction is called base-promoted,rather than base-catalyzed,because a full equivalent of the base is consumed in the reaction. Chapter 22 14
Chapter 22 14 The a Halogenation of Ketones ▪ When a ketone is treated with a halogen and a base, an a halogenation reaction occurs. ▪ The reaction is called base-promoted, rather than base-catalyzed, because a full equivalent of the base is consumed in the reaction

Base-Promoted Halogenation Mechanism enolate ion +H0 Copyright2010 Pearson Prentice Hall,Inc. The base-promoted halogenation takes place by a nucleophilic attack of an enolate ion on the electrophilic halogen molecule. The products are the halogenated ketone and a halide ion. Chapter 22 15
Chapter 22 15 Base-Promoted Halogenation Mechanism ▪ The base-promoted halogenation takes place by a nucleophilic attack of an enolate ion on the electrophilic halogen molecule. ▪ The products are the halogenated ketone and a halide ion