
1.2-and 1.4-Addition to Conjugated Dienes -Electrophilic addition to the double bond produces the most stable intermediate. For conjugated dienes,the intermediate is a resonance-stabilized allylic cation. Nucleophile adds to either Carbon 2 or 4, both of which have the delocalized positive charge. Chapter 15 16
Chapter 15 16 1,2- and 1,4-Addition to Conjugated Dienes ▪ Electrophilic addition to the double bond produces the most stable intermediate. ▪ For conjugated dienes, the intermediate is a resonance-stabilized allylic cation. ▪ Nucleophile adds to either Carbon 2 or 4, both of which have the delocalized positive charge
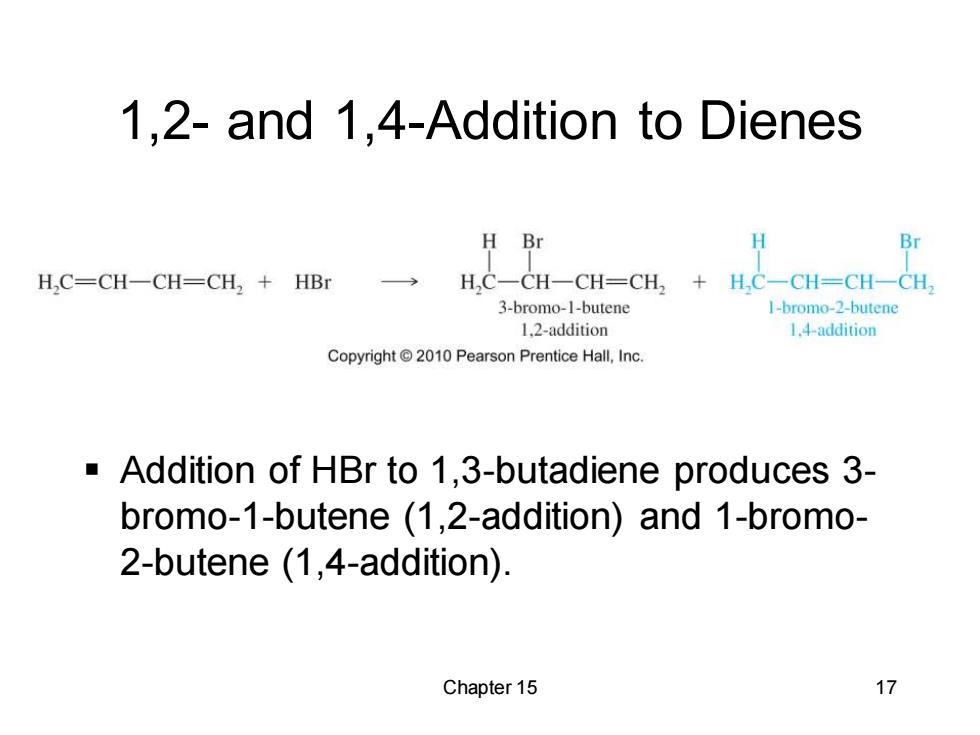
1,2-and 1,4-Addition to Dienes H Br Br H,C=CH一CH=CH2+HBr HC一CH一CH=CH2+H,C一CH=CH-CH 3-bromo-1-butene 1-bromo-2-butene 1.2-addition 1.4-addition Copyright 2010 Pearson Prentice Hall,Inc. Addition of HBr to 1,3-butadiene produces 3- bromo-1-butene (1,2-addition)and 1-bromo- 2-butene (1,4-addition). Chapter 15 17
Chapter 15 17 1,2- and 1,4-Addition to Dienes ▪ Addition of HBr to 1,3-butadiene produces 3- bromo-1-butene (1,2-addition) and 1-bromo- 2-butene (1,4-addition)

Mechanism of 1,2-and 1,4- Addition Step I:Protonation of one of the double bonds forms a resonance-stabilized allylic cation. H H Br allylic cation Copyright2010 Pearson Prentice Hall,Inc. Step 2:A nucleophile attacks at either electrophilic carbon atom 1.2 Br: and 1,2-addition 1.4-addition Copyright 2010 Pearson Prentice Hall,Inc. Chapter 15 18
Chapter 15 18 Mechanism of 1,2- and 1,4- Addition

Kinetic Versus Thermodynamic Control (80%) HC一CH一CH=CH (1,2-product) H Br -80C (20%) H,C- CH=CH一CH (1,4-product) HBr H Br 40℃ H,C=CH一CH=CH2 (15%) H,C一CH一CH=CH, (1,2-product) 40℃ H Br (85%) H,C- CH=CH一CH (1,4-product) H Br Copyright2010 Pearson Prentice Hall,Inc. Chapter 15 19
Chapter 15 19 Kinetic Versus Thermodynamic Control