Molecular Orbitals(MOs) Pi molecular orbitals are the sideways overlap of p orbitals. -p orbitals have two lobes.Plus (+)and minus (-)indicate the opposite phases of the wave function,not electrical charges. When lobes overlap constructively (and + or-and-),a bonding MO is formed. When and lobes overlap,waves cancel out and a node forms;antibonding MO. Chapter 15 6
Chapter 15 6 Molecular Orbitals (MOs) ▪ Pi molecular orbitals are the sideways overlap of p orbitals. ▪ p orbitals have two lobes. Plus (+) and minus (-) indicate the opposite phases of the wave function, not electrical charges. ▪ When lobes overlap constructively (+ and +, or - and -), a bonding MO is formed. ▪ When + and - lobes overlap, waves cancel out and a node forms; antibonding MO

Ethylene Pi MOs (antibonding) destructive energy (bonding)= Copyright2010 Pearson Prentice Hall,Inc The combination of two p orbitals must give two molecular orbitals. Constructive overlap is a bonding MO. Destructive overlap is an antibonding MO. Chapter 15 7
Chapter 15 7 Ethylene Pi MOs ▪ The combination of two p orbitals must give two molecular orbitals. ▪ Constructive overlap is a bonding MO. ▪ Destructive overlap is an antibonding MO

MO for 1,3-Butadiene Lowest energy. bondingbonding bonding ·All bonding interactions. -Electrons are delocalized over four nuclei. Copyright 2010 Pearn Prentice Hall,Inc Chapter 15 8
Chapter 15 8 1 MO for 1,3-Butadiene ▪ Lowest energy. ▪ All bonding interactions. ▪ Electrons are delocalized over four nuclei

2 MO for 1,3-Butadiene Two bonding antibonding bonding interactions. bonding One antibonding interaction. ■A bonding MO. Higher energy than 元1 MO and not as node strong. Chapter 15 9
Chapter 15 9 2 MO for 1,3-Butadiene ▪ Two bonding interactions. ▪ One antibonding interaction. ▪ A bonding MO. ▪ Higher energy than 1 MO and not as strong
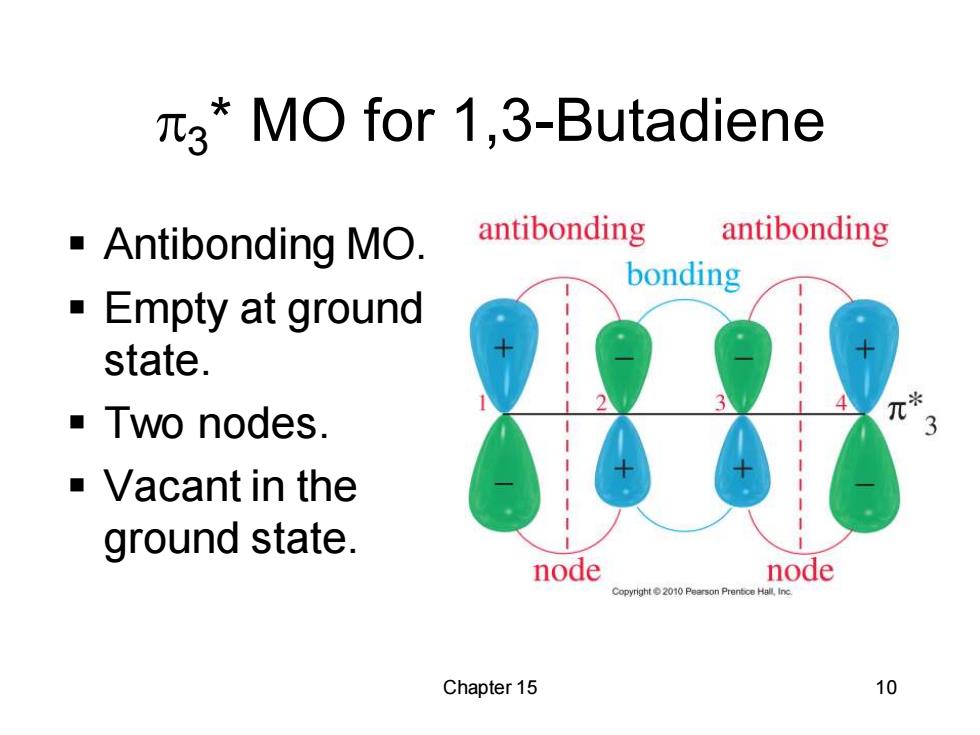
3*MO for 1,3-Butadiene Antibonding MO antibonding antibonding bonding Empty at ground state. Two nodes. Vacant in the ground state. node node Chapter 15 10
Chapter 15 10 3 * MO for 1,3-Butadiene ▪ Antibonding MO. ▪ Empty at ground state. ▪ Two nodes. ▪ Vacant in the ground state