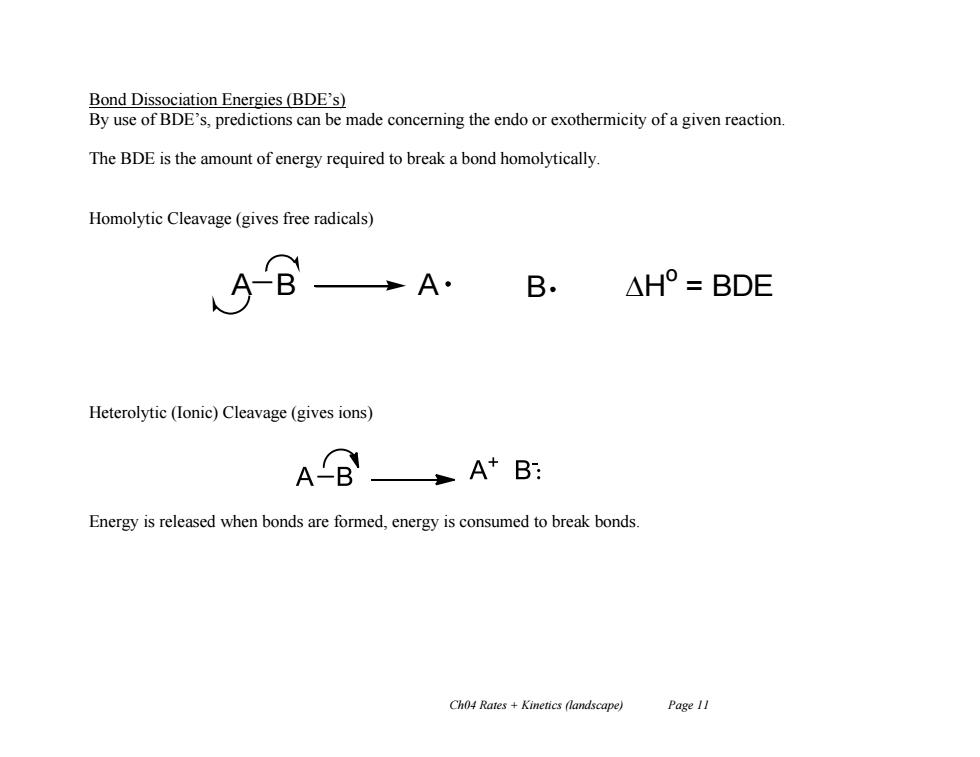
Bond Dissociation Energies(BDE's) By use of BDE's,predictions can be made concerning the endo or exothermicity of a given reaction. The BDE is the amount of energy required to break a bond homolytically. Homolytic Cleavage(gives free radicals) A· B △H°=BDE Heterolytic (Ionic)Cleavage(gives ions) AB A+B: Energy is released when bonds are formed,energy is consumed to break bonds. Ch04 Rates Kinetics (landscape) Page 11
Ch04 Rates + Kinetics (landscape) Page 11 Bond Dissociation Energies (BDE’s) By use of BDE’s, predictions can be made concerning the endo or exothermicity of a given reaction. The BDE is the amount of energy required to break a bond homolytically. Homolytic Cleavage (gives free radicals) Heterolytic (Ionic) Cleavage (gives ions) Energy is released when bonds are formed, energy is consumed to break bonds. A B A B H o = BDE
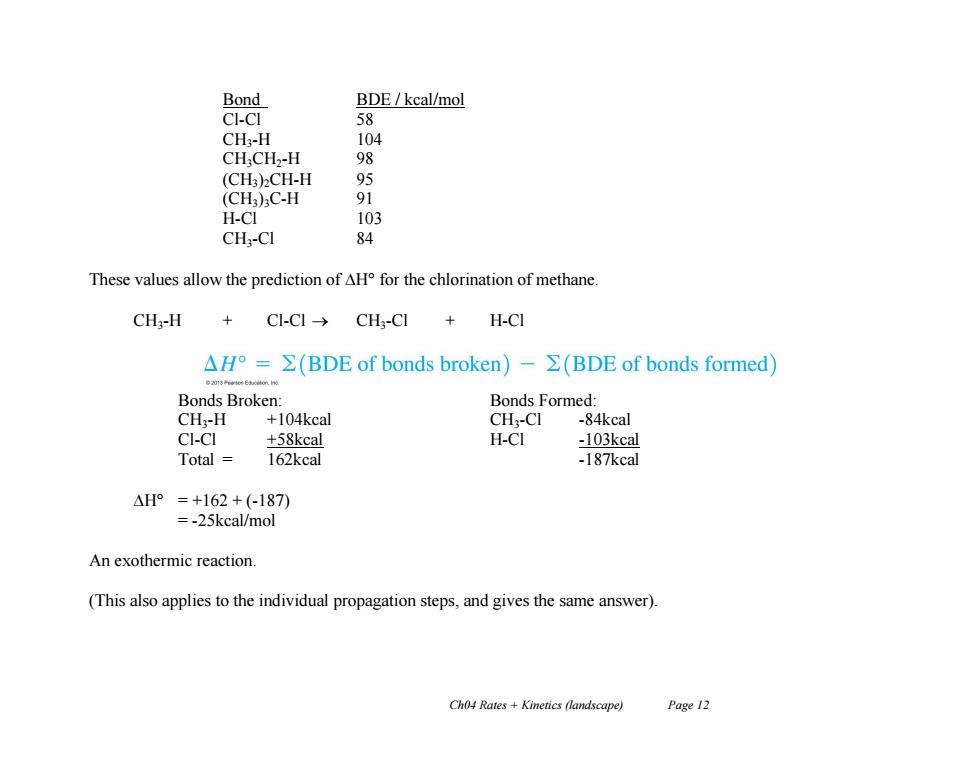
Bond BDE/kcal/mol CI-CI 58 CH:-H 104 CHCH2-H 98 (CH3)2CH-H 95 (CH3):C-H 91 H-CI 103 CH:-CI 84 These values allow the prediction of AH for the chlorination of methane. CH-H + C1-C1→CH3-C1+H-CI △H°=∑(BDE of bonds broken)-Σ(BDE of bonds formed) Bonds Broken: Bonds Formed: CH:-H +104kcal CH2-CI -84kcal CI-CI +58kcal H-CI -103kcal Total 162kcal -187kcal △H°=+162+(-187) =-25kcal/mol An exothermic reaction. (This also applies to the individual propagation steps,and gives the same answer). Ch04 Rates Kinetics (landscape) Page 12
Ch04 Rates + Kinetics (landscape) Page 12 Bond BDE / kcal/mol Cl-Cl 58 CH3-H 104 CH3CH2-H 98 (CH3)2CH-H 95 (CH3)3C-H 91 H-Cl 103 CH3-Cl 84 These values allow the prediction of H° for the chlorination of methane. CH3-H + Cl-Cl CH3-Cl + H-Cl Bonds Broken: Bonds Formed: CH3-H +104kcal CH3-Cl -84kcal Cl-Cl +58kcal H-Cl -103kcal Total = 162kcal -187kcal H° = +162 + (-187) = -25kcal/mol An exothermic reaction. (This also applies to the individual propagation steps, and gives the same answer)

Kinetics and the Rate Equation The study of reaction rates is called kinetics. The rate of a reaction is just as important as the position of equilibrium. (Just because a reaction has a favorable AG,does not mean the reaction will spontaneously go) The rate of a reaction is how fast the products appear and how slow the reactants disappear.These can be obtained experimentally by monitoring the concentrations of either the products or reactants with respect to time. Reaction rates depend on the concentrations of reactants since at higher concentrations,collisions between reactants (leading to a reaction)are more likely. The rate equation(rate law)relates the concentrations of the reactants to the rate of reaction. Consider:A+B->C+D The rate is proportional to the concentrations of A and B raised to some power (a and b). rate kr[A][B]5 Where k,is a rate constant,and a and b have to be determined experimentally Values a and b are usually whole numbers,and are called the order of the reaction. (a+b)is the overall order of a reaction. Ch04 Rates +Kinetics (landscape) Page 13
Ch04 Rates + Kinetics (landscape) Page 13 Kinetics and the Rate Equation The study of reaction rates is called kinetics. The rate of a reaction is just as important as the position of equilibrium. (Just because a reaction has a favorable G°, does not mean the reaction will spontaneously go). The rate of a reaction is how fast the products appear and how slow the reactants disappear. These can be obtained experimentally by monitoring the concentrations of either the products or reactants with respect to time. Reaction rates depend on the concentrations of reactants since at higher concentrations, collisions between reactants (leading to a reaction) are more likely. The rate equation (rate law) relates the concentrations of the reactants to the rate of reaction. Consider: A + B C + D The rate is proportional to the concentrations of A and B raised to some power (a and b). Where kr is a rate constant, and a and b have to be determined experimentally. Values a and b are usually whole numbers, and are called the order of the reaction. (a+b) is the overall order of a reaction