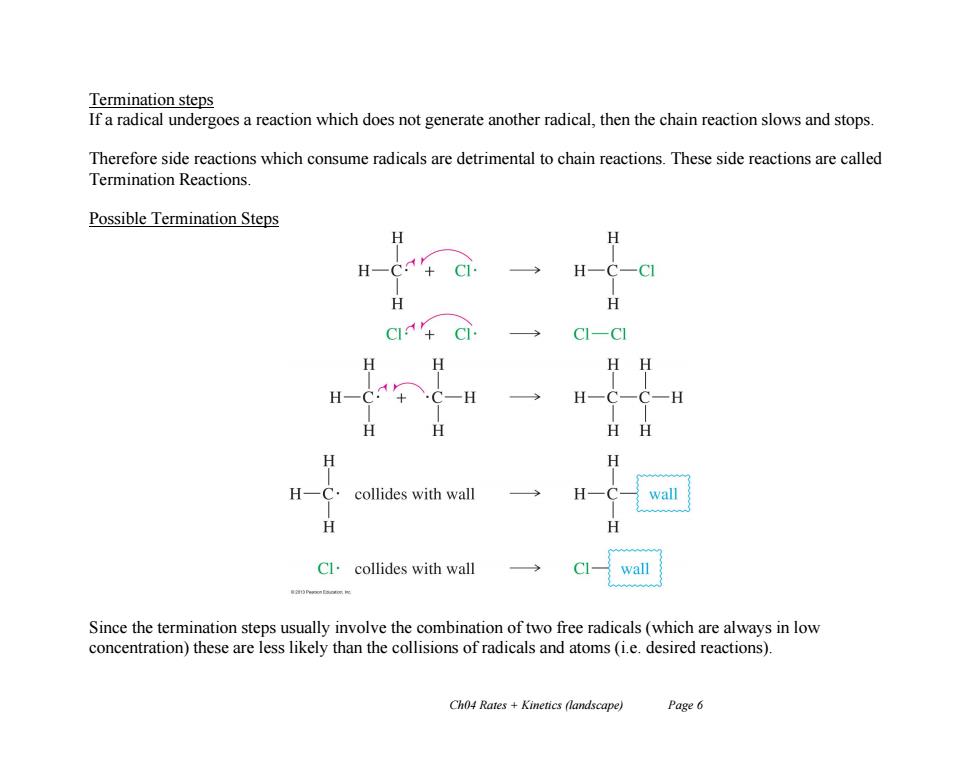
Termination steps If a radical undergoes a reaction which does not generate another radical,then the chain reaction slows and stops. Therefore side reactions which consume radicals are detrimental to chain reactions.These side reactions are called Termination Reactions. Possible Termination Steps H H-C H H -C] H HH H collides with wall wall Cl.collides with wall CI- wall Since the termination steps usually involve the combination of two free radicals(which are always in low concentration)these are less likely than the collisions of radicals and atoms (i.e.desired reactions). Ch04 Rates Kinetics (landscape) Page 6
Ch04 Rates + Kinetics (landscape) Page 6 Termination steps If a radical undergoes a reaction which does not generate another radical, then the chain reaction slows and stops. Therefore side reactions which consume radicals are detrimental to chain reactions. These side reactions are called Termination Reactions. Possible Termination Steps Since the termination steps usually involve the combination of two free radicals (which are always in low concentration) these are less likely than the collisions of radicals and atoms (i.e. desired reactions)
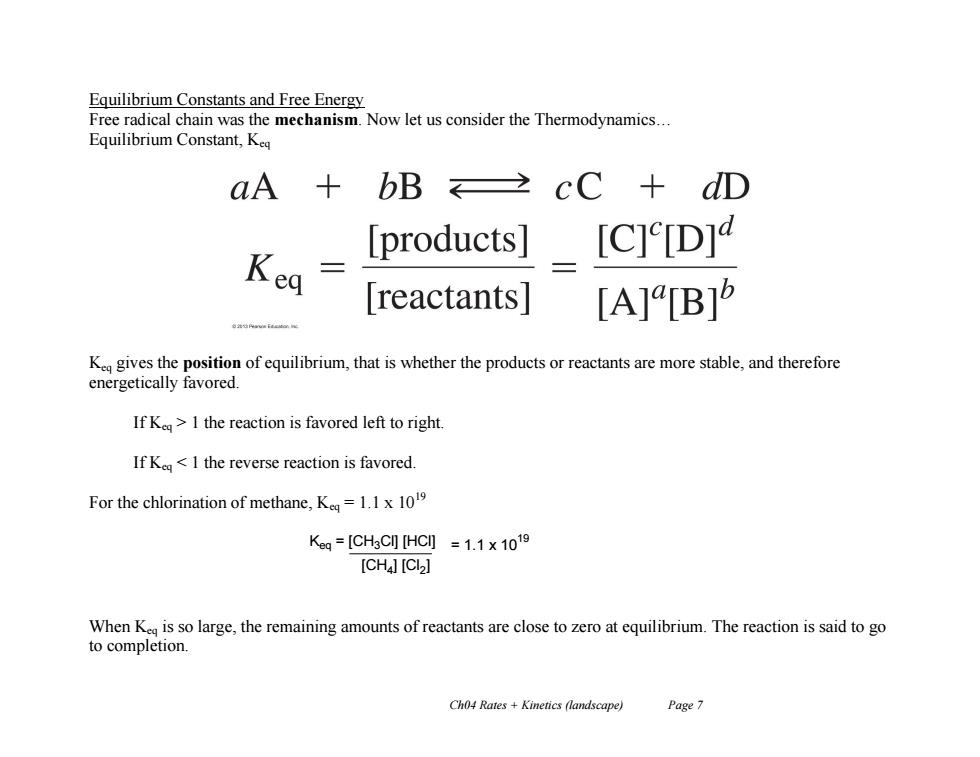
Equilibrium Constants and Free Energy Free radical chain was the mechanism.Now let us consider the Thermodynamics... Equilibrium Constant,Keq aA bB cC+dD products] [C]e[D] Kea reactants| [A][B]5 K gives the position of equilibrium,that is whether the products or reactants are more stable,and therefore energetically favored. If Ke>I the reaction is favored left to right. If Kea<1 the reverse reaction is favored. For the chlorination of methane,K=1.1x10 Kg=[CH3CHC]=1.1×1019 [CH4][CI>] When K is so large,the remaining amounts of reactants are close to zero at equilibrium.The reaction is said to go to completion. Ch04 Rates Kinetics (landscape) Page 7
Ch04 Rates + Kinetics (landscape) Page 7 Equilibrium Constants and Free Energy Free radical chain was the mechanism. Now let us consider the Thermodynamics… Equilibrium Constant, Keq Keq gives the position of equilibrium, that is whether the products or reactants are more stable, and therefore energetically favored. If Keq > 1 the reaction is favored left to right. If Keq < 1 the reverse reaction is favored. For the chlorination of methane, Keq = 1.1 x 1019 When Keq is so large, the remaining amounts of reactants are close to zero at equilibrium. The reaction is said to go to completion. Keq = [CH3Cl] [HCl] [CH4 ] [Cl2 ] = 1.1 x 10 19
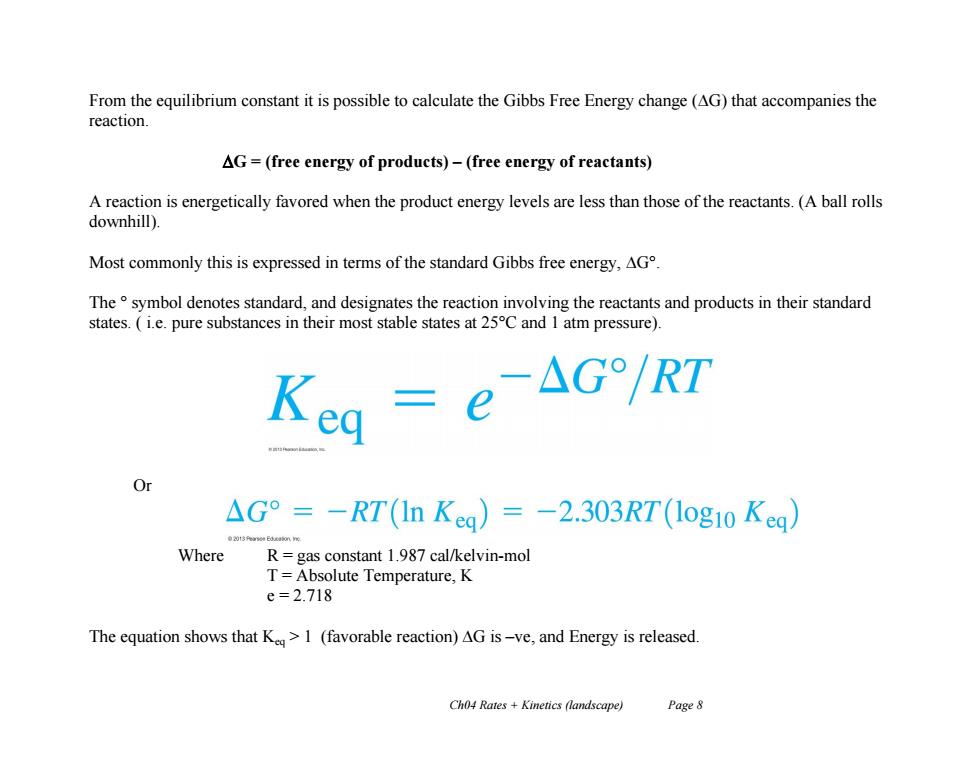
From the equilibrium constant it is possible to calculate the Gibbs Free Energy change (AG)that accompanies the reaction. AG=(free energy of products)-(free energy of reactants) A reaction is energetically favored when the product energy levels are less than those of the reactants.(A ball rolls downhill). Most commonly this is expressed in terms of the standard Gibbs free energy,AG The symbol denotes standard,and designates the reaction involving the reactants and products in their standard states.(ie.pure substances in their most stable states at 25C and 1 atm pressure). △G°=-RT(In Keg)=-2.303RT(1og10Kea) Where R=gas constant 1.987 cal/kelvin-mol T=Absolute Temperature,K e=2.718 The equation shows that K>1 (favorable reaction)AG is-ve,and Energy is released. Ch04 Rates Kinetics (landscape) Page 8
Ch04 Rates + Kinetics (landscape) Page 8 From the equilibrium constant it is possible to calculate the Gibbs Free Energy change (G) that accompanies the reaction. G = (free energy of products) – (free energy of reactants) A reaction is energetically favored when the product energy levels are less than those of the reactants. (A ball rolls downhill). Most commonly this is expressed in terms of the standard Gibbs free energy, G°. The ° symbol denotes standard, and designates the reaction involving the reactants and products in their standard states. ( i.e. pure substances in their most stable states at 25°C and 1 atm pressure). Or Where R = gas constant 1.987 cal/kelvin-mol T = Absolute Temperature, K e = 2.718 The equation shows that Keq > 1 (favorable reaction) G is –ve, and Energy is released

Enthalpy and Entropy There are two factors that combine to give the free energy change for a reaction:Enthalpy(H)and Entropy(S). △G°=△H°.T△S° AH=change in enthalpy=(enthalpy of prods)-(enthalpy of reactants) AS=change in entropy =(entropy of prods)-(entropy of reactants) (Typically the AH term>>TAS term) Enthalpy The change in enthalpy is the heat evolved or consumed in a reaction,normally in kcal/mol. Reactions tend to favor products with stronger bonds(i.e.lower enthalpy). AH is-ve,exothermic reaction,heat is evolved,stronger bonds are formed. AH is +ve,endothermic reaction,heat is consumed,weaker bonds are formed. A-ve AH is a favorable contribution to the free energy change. A +ve AH is an unfavorable contribution to the free energy change The AH for chlorination of methane is-25kcal/mol.(Very exothermic) Ch04 Rates Kinetics (landscape) Page 9
Ch04 Rates + Kinetics (landscape) Page 9 Enthalpy and Entropy There are two factors that combine to give the free energy change for a reaction: Enthalpy (H) and Entropy (S). G° = H° - TS° H° = change in enthalpy = (enthalpy of prods) – (enthalpy of reactants) S° = change in entropy = (entropy of prods) – (entropy of reactants) (Typically the H term >> TS term) Enthalpy The change in enthalpy is the heat evolved or consumed in a reaction, normally in kcal/mol. Reactions tend to favor products with stronger bonds (i.e. lower enthalpy). H is –ve, exothermic reaction, heat is evolved, stronger bonds are formed. H is +ve, endothermic reaction, heat is consumed, weaker bonds are formed. A –ve H is a favorable contribution to the free energy change. A +ve H is an unfavorable contribution to the free energy change. The H° for chlorination of methane is –25kcal/mol. (Very exothermic)

Entropy Entropy is often described as randomness,or freedom to move,or disorder. Reactions tend to favor products with greatest entropy Note the sign of the entropy contribution to AG: A +ve AS term makes a favorable contribution to the free energy. For the chlorination of methane,AS=2.9 entropy units(eu) So -T△S°=-298Kx2.9=-0.86kcal/mol Remember△H°=-25kcal,(much larger) So△G°=-25-0.86=-26kcal/mol (Often AG is approximated to be equal to AH). In kJ... △G°=△H°-T△S°=-105.1kJ/mol-3.61kJ/mol =-108.7 kJ/mol (-25.9 kcal/mol) Ch04 Rates +Kinetics (landscape) Page 10
Ch04 Rates + Kinetics (landscape) Page 10 Entropy Entropy is often described as randomness, or freedom to move, or disorder. Reactions tend to favor products with greatest entropy. Note the sign of the entropy contribution to G: A +ve S term makes a favorable contribution to the free energy. For the chlorination of methane, S° = 2.9 entropy units (eu) So -TS° = -298K x 2.9 = -0.86kcal/mol Remember H° = -25kcal, (much larger) So G° = -25 - 0.86 =-26kcal/mol. (Often G is approximated to be equal to H). In kJ…