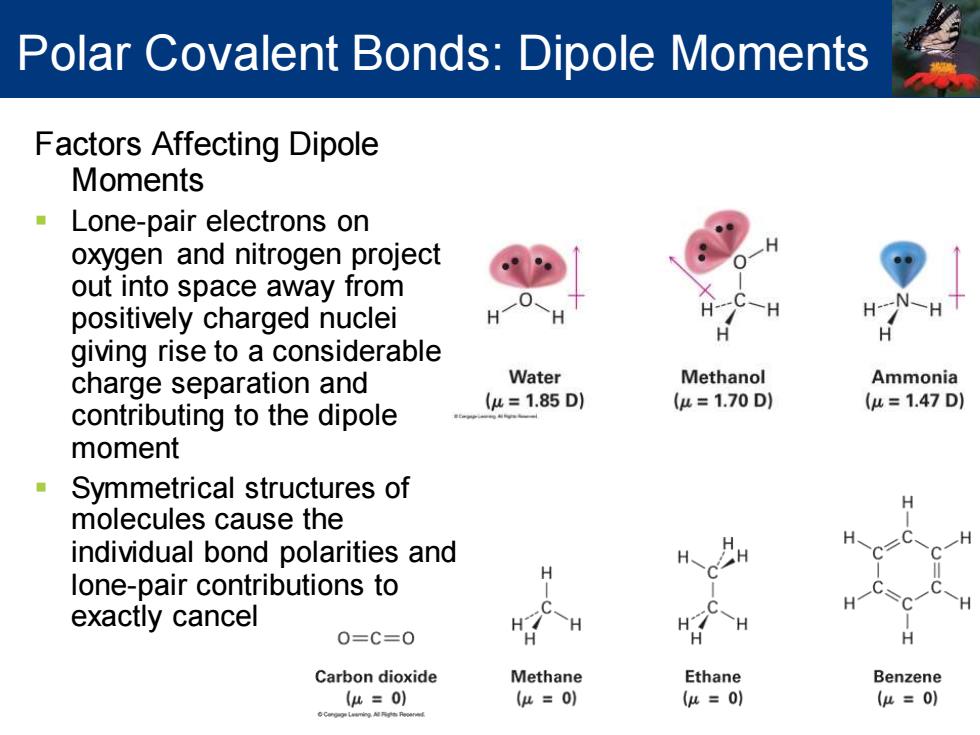
Polar Covalent Bonds:Dipole Moments Factors Affecting Dipole Moments Lone-pair electrons on oxygen and nitrogen project out into space away from positively charged nuclei giving rise to a considerable charge separation and Water Methanol Ammonia contributing to the dipole (u=1.85D) (u=1.70D1 (u=1.47D) moment Symmetrical structures of molecules cause the individual bond polarities and lone-pair contributions to H exactly cancel H H 0=C=0 H Carbon dioxide Methane Ethane Benzene u=0) (μ=0) (u=0) (μ=0)
Polar Covalent Bonds: Dipole Moments Factors Affecting Dipole Moments ▪ Lone-pair electrons on oxygen and nitrogen project out into space away from positively charged nuclei giving rise to a considerable charge separation and contributing to the dipole moment ▪ Symmetrical structures of molecules cause the individual bond polarities and lone-pair contributions to exactly cancel

Worked Example 2.1 Predicting the Direction of a Dipole Moment Make a three-dimensional drawing of methylamine, CH3NH2,and show the direction of its dipole moment (1.31)
Make a three-dimensional drawing of methylamine, CH3NH2 , and show the direction of its dipole moment ( = 1.31) Worked Example 2.1 Predicting the Direction of a Dipole Moment

Worked Example 2.1 Predicting the Direction of a Dipole Moment Strategy Look for any lone-pair electrons Identify any atom with an electronegativity substantially different from that of carbon (usually O, N,F,Cl,orBr) Electron density will be displaced in the general direction of the electronegative atoms and the lone pairs
Strategy ▪ Look for any lone-pair electrons ▪ Identify any atom with an electronegativity substantially different from that of carbon (usually O, N, F, Cl, or Br) ▪ Electron density will be displaced in the general direction of the electronegative atoms and the lone pairs Worked Example 2.1 Predicting the Direction of a Dipole Moment
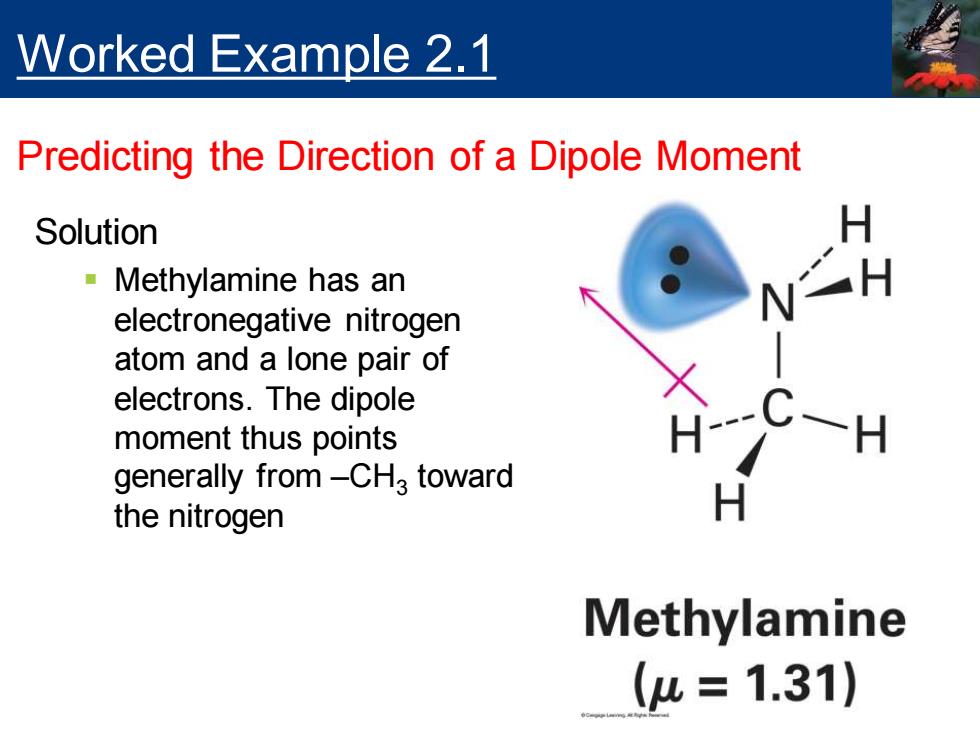
Worked Example 2.1 Predicting the Direction of a Dipole Moment Solution Methylamine has an electronegative nitrogen atom and a lone pair of electrons.The dipole moment thus points generally from-CHa toward the nitrogen H Methylamine (u=1.31)
Solution ▪ Methylamine has an electronegative nitrogen atom and a lone pair of electrons. The dipole moment thus points generally from –CH3 toward the nitrogen Worked Example 2.1 Predicting the Direction of a Dipole Moment
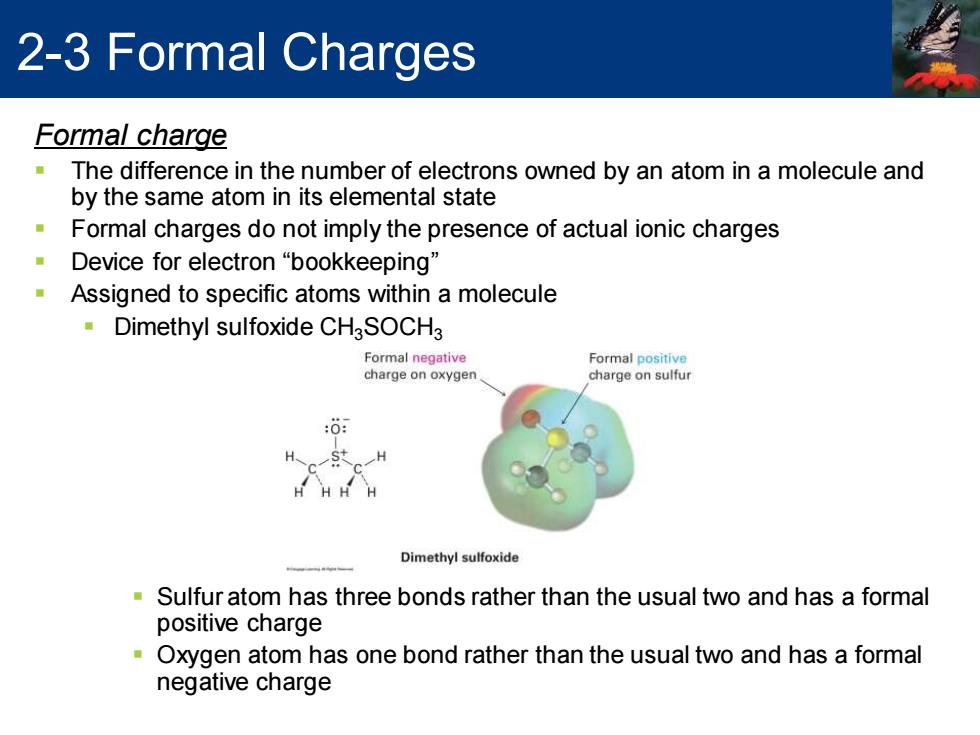
2-3 Formal Charges Formal charge The difference in the number of electrons owned by an atom in a molecule and by the same atom in its elemental state Formal charges do not imply the presence of actual ionic charges Device for electron "bookkeeping" Assigned to specific atoms within a molecule Dimethyl sulfoxide CHgSOCH3 Formal negative Formal positive charge on oxygen charge on sulfur :0 H HHH Dimethyl sulfoxide Sulfur atom has three bonds rather than the usual two and has a formal positive charge Oxygen atom has one bond rather than the usual two and has a formal negative charge
2-3 Formal Charges Formal charge ▪ The difference in the number of electrons owned by an atom in a molecule and by the same atom in its elemental state ▪ Formal charges do not imply the presence of actual ionic charges ▪ Device for electron “bookkeeping” ▪ Assigned to specific atoms within a molecule ▪ Dimethyl sulfoxide CH3SOCH3 ▪ Sulfur atom has three bonds rather than the usual two and has a formal positive charge ▪ Oxygen atom has one bond rather than the usual two and has a formal negative charge