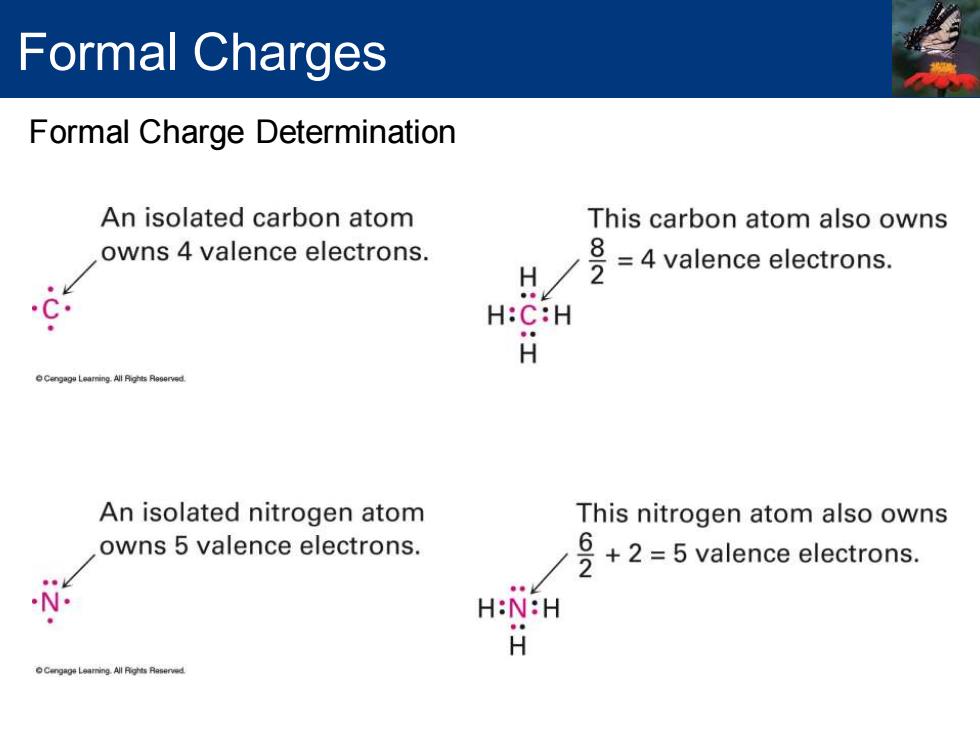
Formal Charges Formal Charge Determination An isolated carbon atom This carbon atom also owns owns 4 valence electrons. H 号=4 valence electrons C H:C:H H An isolated nitrogen atom This nitrogen atom also owns owns 5 valence electrons. 2-5 valence electrons. H:N:H H
Formal Charges Formal Charge Determination

Formal Charges Number of Number of Formal charge valence electrons valence electrons in free atom in bonded atom Number of Number of Number of valence electrons bonding electrons nonbonding in free atom 2 electrons For sulfur: Sulfur valence electrons =6 Sulfur bonding electrons =6 Sulfur nonbonding electrons =2 Formal charge=6-6/2-2 =+1 For oxygen: Oxygen valence electrons =6 Oxygen bonding electrons =2 Oxygen nonbonding electrons =6 Formal charge=6-2/2-6 =-1
Formal Charges

Formal Charges TABLE2.2 A Summary of Common Formal Charges Atom N 0 Structure Valence electrons Number of 3 bonds Number of 0 nonbonding electrons Formal charge +1 +1 +1
Formal Charges
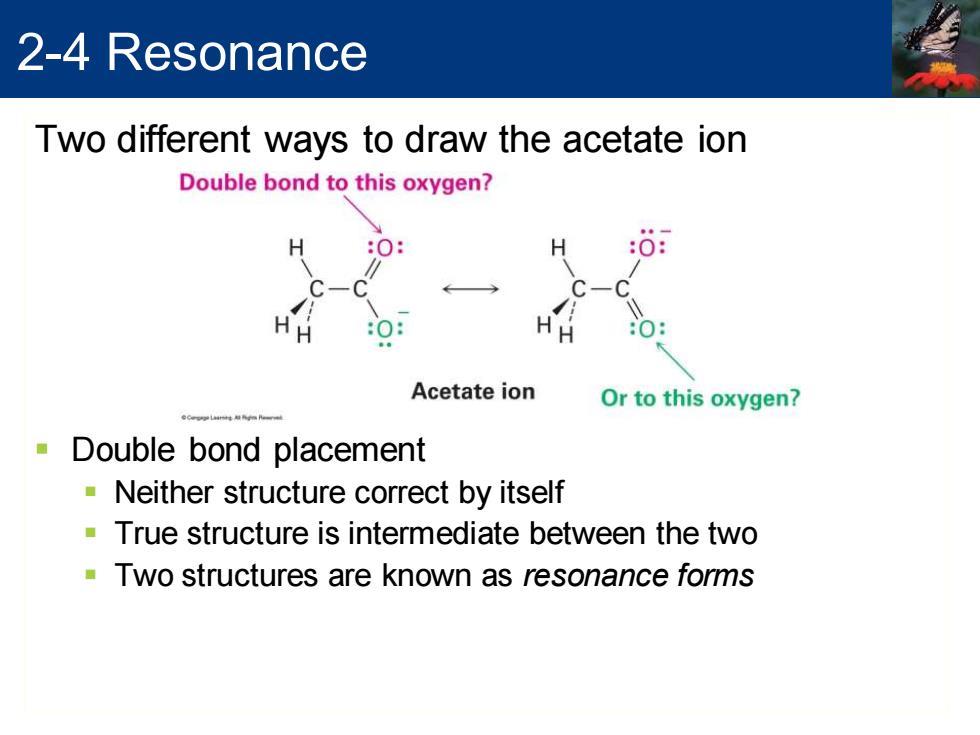
2-4 Resonance Two different ways to draw the acetate ion Double bond to this oxygen? :0 HH Acetate ion Or to this oxygen? Double bond placement Neither structure correct by itself True structure is intermediate between the two Two structures are known as resonance forms
Two different ways to draw the acetate ion ▪ Double bond placement ▪ Neither structure correct by itself ▪ True structure is intermediate between the two ▪ Two structures are known as resonance forms 2-4 Resonance
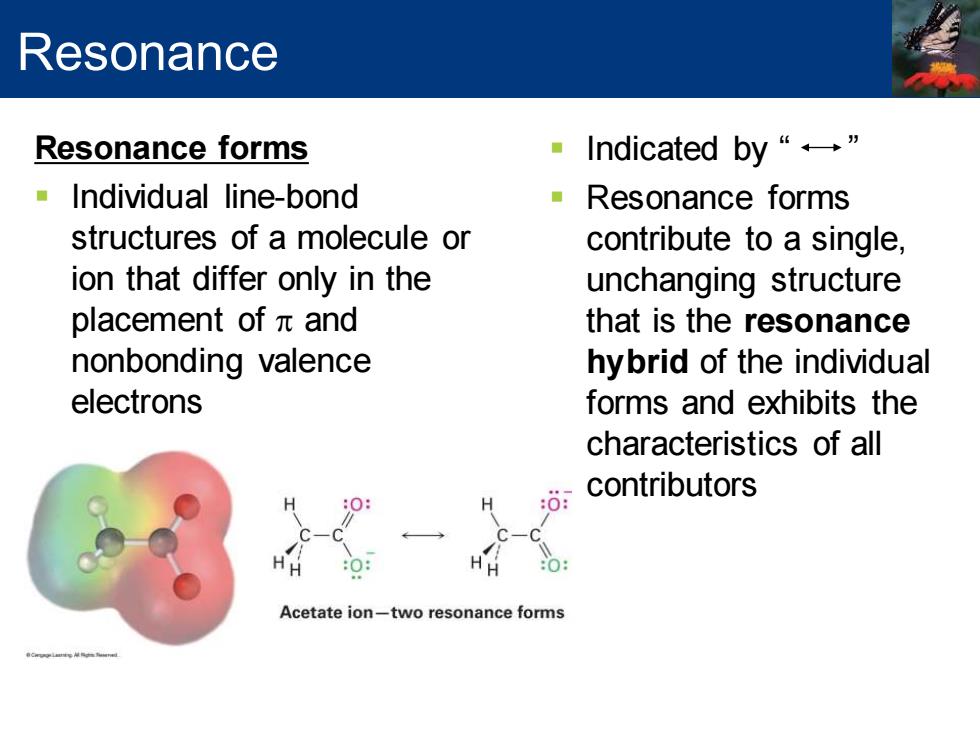
Resonance Resonance forms Indicated by“←” Individual line-bond ·Resonance forms structures of a molecule or contribute to a single, ion that differ only in the unchanging structure placement ofπand that is the resonance nonbonding valence hybrid of the individual electrons forms and exhibits the characteristics of all contributors H H C-0 C-C Acetate ion-two resonance forms
Resonance forms ▪ Individual line-bond structures of a molecule or ion that differ only in the placement of p and nonbonding valence electrons ▪ Indicated by “ ” ▪ Resonance forms contribute to a single, unchanging structure that is the resonance hybrid of the individual forms and exhibits the characteristics of all contributors Resonance