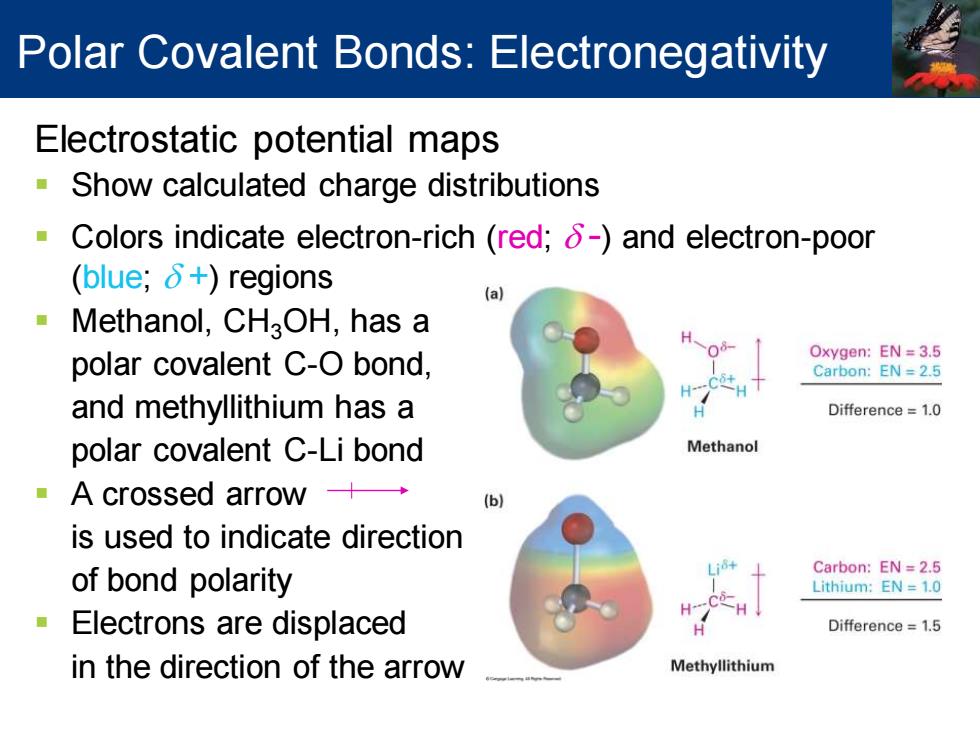
Polar Covalent Bonds:Electronegativity Electrostatic potential maps Show calculated charge distributions Colors indicate electron-rich (red;8-)and electron-poor (blue;δ+)regions (a Methanol,CHaOH,has a polar covalent C-O bond, Oxygen:EN=3.5 Carbon:EN=2.5 H and methyllithium has a Difference=1.0 polar covalent C-Li bond Methanol A crossed arrow+→ is used to indicate direction Carbon:EN 2.5 of bond polarity Lithium:EN 1.0 Electrons are displaced Difference=1.5 in the direction of the arrow Methyllithium
Electrostatic potential maps ▪ Show calculated charge distributions ▪ Colors indicate electron-rich (red; d -) and electron-poor (blue; d +) regions ▪ Methanol, CH3OH, has a polar covalent C-O bond, and methyllithium has a polar covalent C-Li bond ▪ A crossed arrow is used to indicate direction of bond polarity ▪ Electrons are displaced in the direction of the arrow Polar Covalent Bonds: Electronegativity
Polar Covalent Bonds:Electronegativity An atom's ability to polarize a bond is known as the inductive effect Inductive effect The electron-attracting or electron-withdrawing effect transmitted through o bonds.Electronegative elements have an electron-withdrawing inductive effect Metals inductively donate electrons Reactive nonmetals inductively withdraw electrons Inductive effects play a major role in understanding chemical reactivity
An atom’s ability to polarize a bond is known as the inductive effect Inductive effect ▪ The electron-attracting or electron-withdrawing effect transmitted through s bonds. Electronegative elements have an electron-withdrawing inductive effect ▪ Metals inductively donate electrons ▪ Reactive nonmetals inductively withdraw electrons ▪ Inductive effects play a major role in understanding chemical reactivity Polar Covalent Bonds: Electronegativity
2-2 Polar Covalent Bonds:Dipole Moments Molecules as a whole are often polar 日 Molecular polarity results from the vector summation of all individual bond polarities and lone-pair contributions in the molecule Strongly polar substances are soluble in polar solvents like water Dipole moment ( Magnitude of charge Q at either end of molecular dipole times distance r between charges u=oxr,in debyes (D) 1D=3.336×10-30 coulomb meter(C·m) A measure of the net polarity of a molecule Arises when the centers of mass of positive and negative charges within a molecule do not coincide
Molecules as a whole are often polar ▪ Molecular polarity results from the vector summation of all individual bond polarities and lone-pair contributions in the molecule ▪ Strongly polar substances are soluble in polar solvents like water Dipole moment () ▪ Magnitude of charge Q at either end of molecular dipole times distance r between charges ▪ = Q r, in debyes (D) 1 D = 3.336 10−30 coulomb meter (C • m) ▪ A measure of the net polarity of a molecule ▪ Arises when the centers of mass of positive and negative charges within a molecule do not coincide 2-2 Polar Covalent Bonds: Dipole Moments
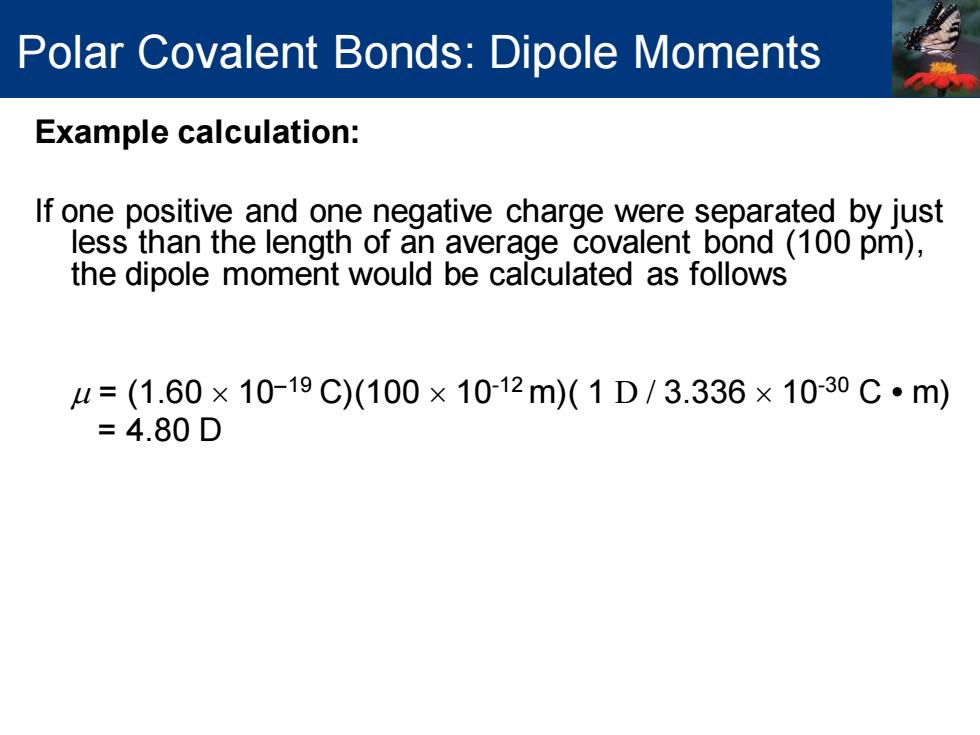
Polar Covalent Bonds:Dipole Moments Example calculation: If one positive and one negative charge were separated by just less than the length of an average covalent bond (100 pm), the dipole moment would be calculated as follows =(1.60×10-19C)(100×1012m)(1D/3.336×10-30C·m) =4.80D
Example calculation: If one positive and one negative charge were separated by just less than the length of an average covalent bond (100 pm), the dipole moment would be calculated as follows = (1.60 10−19 C)(100 10-12 m)( 1 D / 3.336 10-30 C • m) = 4.80 D Polar Covalent Bonds: Dipole Moments
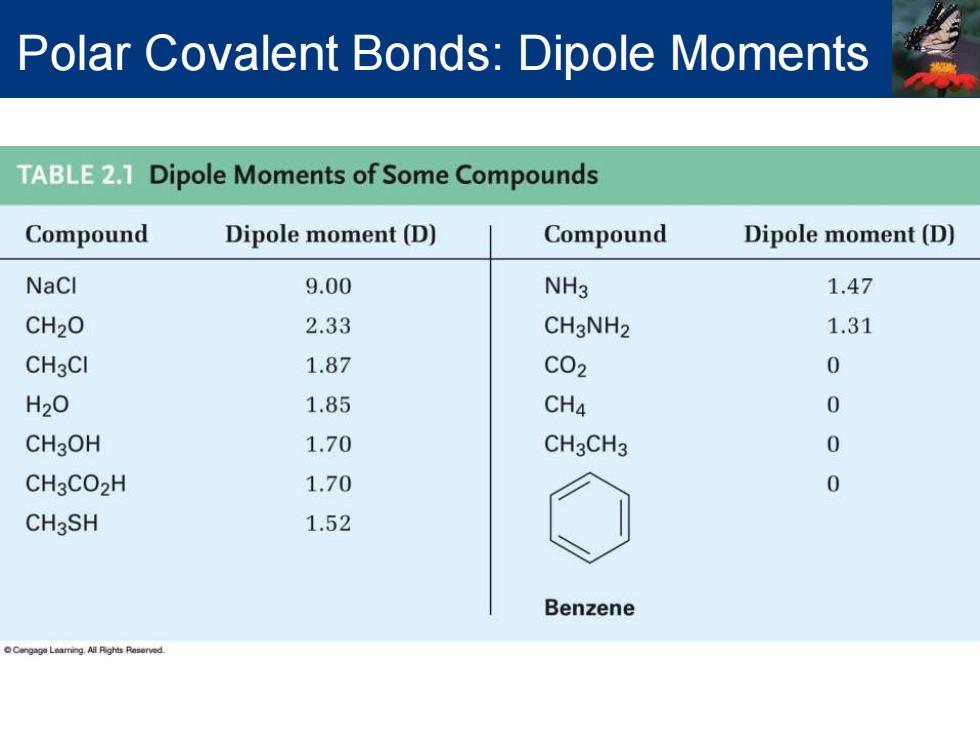
Polar Covalent Bonds:Dipole Moments TABLE2.1 Dipole Moments of Some Compounds Compound Dipole moment(D) Compound Dipole moment(D) NaCl 9.00 NH3 1.47 CH20 2.33 CH3NH2 1.31 CH3Cl 1.87 C02 0 H20 1.85 CH4 0 CH3OH 1.70 CH3CH3 0 CH3CO2H 1.70 0 CH3SH 1.52 Benzene
Polar Covalent Bonds: Dipole Moments