
Soap Crude soaps are purified by adding NaCl or KCI to precipitate the pure carboxylate salts Smooth soap that precipitates is dried,perfumed,and pressed into bars for household use Soaps act as cleansers because the two ends of a soap molecule are different The carboxylate end of the long-chain molecule is ionic and therefore hydrophilic The long hydrocarbon portion of the molecule is nonpolar and hydrophobic The net effect of these two opposing tendencies is that soaps are attracted to both oils and water and therefore useful as cleansers
Crude soaps are purified by adding NaCl or KCl to precipitate the pure carboxylate salts ▪ Smooth soap that precipitates is dried, perfumed, and pressed into bars for household use ▪ Soaps act as cleansers because the two ends of a soap molecule are different ▪ The carboxylate end of the long-chain molecule is ionic and therefore hydrophilic ▪ The long hydrocarbon portion of the molecule is nonpolar and hydrophobic ▪ The net effect of these two opposing tendencies is that soaps are attracted to both oils and water and therefore useful as cleansers Soap

Soap When soaps are dispersed in water they form micelles,molecular spheres with both hydrophobic and hydrophilic properties The long hydrophobic tails cluster together on the hydrophobic inside of the spheres The ionic heads group together on the hydrophilic surface of the spheres out into the water Grease and oil droplets are solubilized in water when they are coated by the nonpolar tails of soap molecules in the center of the micelles Ionic head Water Hydrocarbon tail
When soaps are dispersed in water they form micelles, molecular spheres with both hydrophobic and hydrophilic properties ▪ The long hydrophobic tails cluster together on the hydrophobic inside of the spheres ▪ The ionic heads group together on the hydrophilic surface of the spheres out into the water ▪ Grease and oil droplets are solubilized in water when they are coated by the nonpolar tails of soap molecules in the center of the micelles Soap
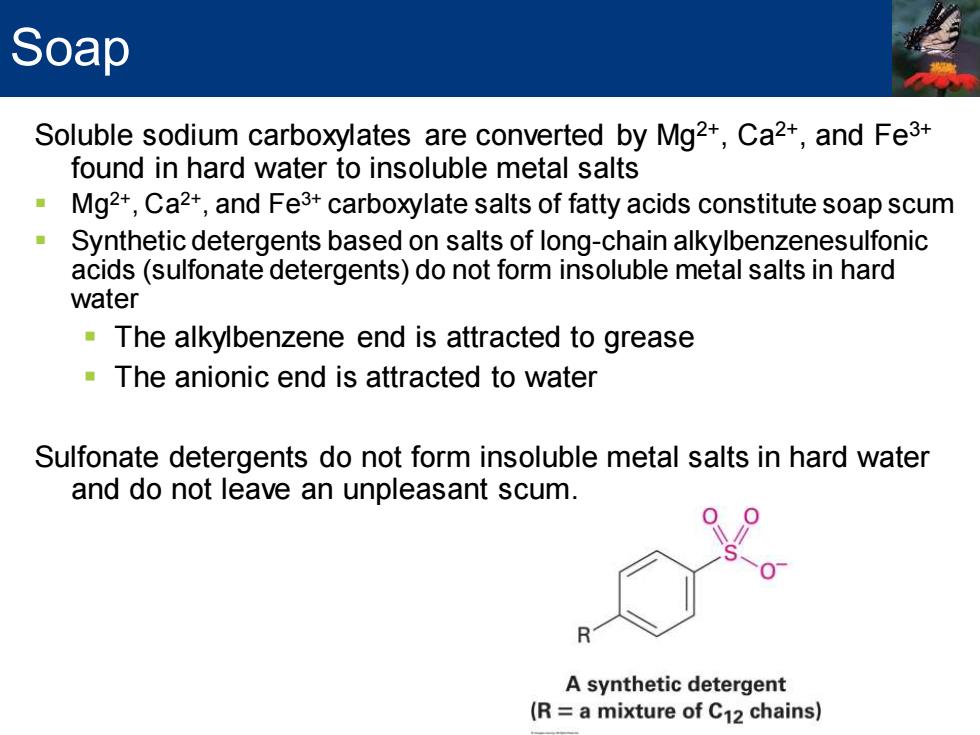
Soap Soluble sodium carboxylates are converted by Mg2+,Ca2+,and Fe3+ found in hard water to insoluble metal salts Mg2+,Ca2+,and Fe3+carboxylate salts of fatty acids constitute soap scum Synthetic detergents based on salts of long-chain alkylbenzenesulfonic acids(sulfonate detergents)do not form insoluble metal salts in hard water The alkylbenzene end is attracted to grease The anionic end is attracted to water Sulfonate detergents do not form insoluble metal salts in hard water and do not leave an unpleasant scum. R A synthetic detergent (R a mixture of C12 chains)
Soluble sodium carboxylates are converted by Mg2+, Ca2+, and Fe3+ found in hard water to insoluble metal salts ▪ Mg2+, Ca2+, and Fe3+ carboxylate salts of fatty acids constitute soap scum ▪ Synthetic detergents based on salts of long-chain alkylbenzenesulfonic acids (sulfonate detergents) do not form insoluble metal salts in hard water ▪ The alkylbenzene end is attracted to grease ▪ The anionic end is attracted to water Sulfonate detergents do not form insoluble metal salts in hard water and do not leave an unpleasant scum. Soap

23-3 Phospholipids Phospholipids are diesters of phosphoric acid,HaPO 0 HO R- O-R R HO HO A carboxylic B" acid ester A phosphoric A phosphoric A phosphoric acid monoester acid diester acid triester
Phospholipids are diesters of phosphoric acid, H3PO4 23-3 Phospholipids
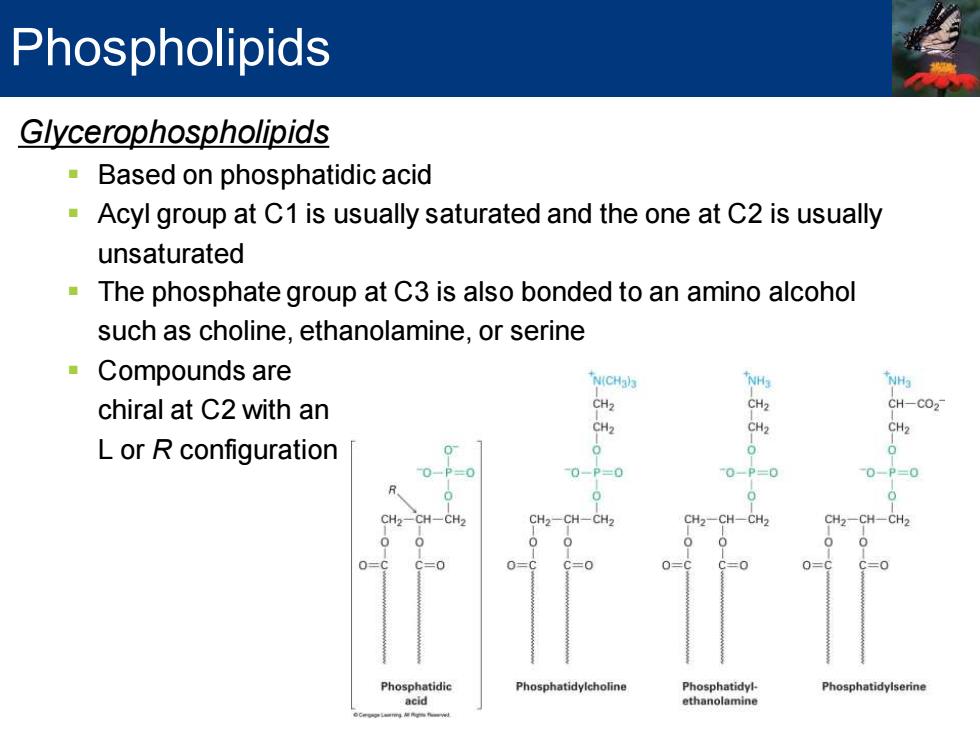
Phospholipids Glycerophospholipids Based on phosphatidic acid Acyl group at C1 is usually saturated and the one at C2 is usually unsaturated The phosphate group at C3 is also bonded to an amino alcohol such as choline,ethanolamine,or serine Compounds are N(CHal3 NH 'NHg chiral at C2 with an CH2 CH2 CH-CO2- CH2 CH2 CH2 L or R configuration 0 0- 0-P=0 0-P=0 0-P=0 CH2-CH-CH2 CH2-CH-CH2 CH2-CH-CH2 CH2-CH-CH2 0 0=C C=0 0= 0 Phosphatidic Phosphatidylcholine Phosphatidyl- Phosphatidylserine acid ethanolamine
Glycerophospholipids ▪ Based on phosphatidic acid ▪ Acyl group at C1 is usually saturated and the one at C2 is usually unsaturated ▪ The phosphate group at C3 is also bonded to an amino alcohol such as choline, ethanolamine, or serine ▪ Compounds are chiral at C2 with an L or R configuration Phospholipids