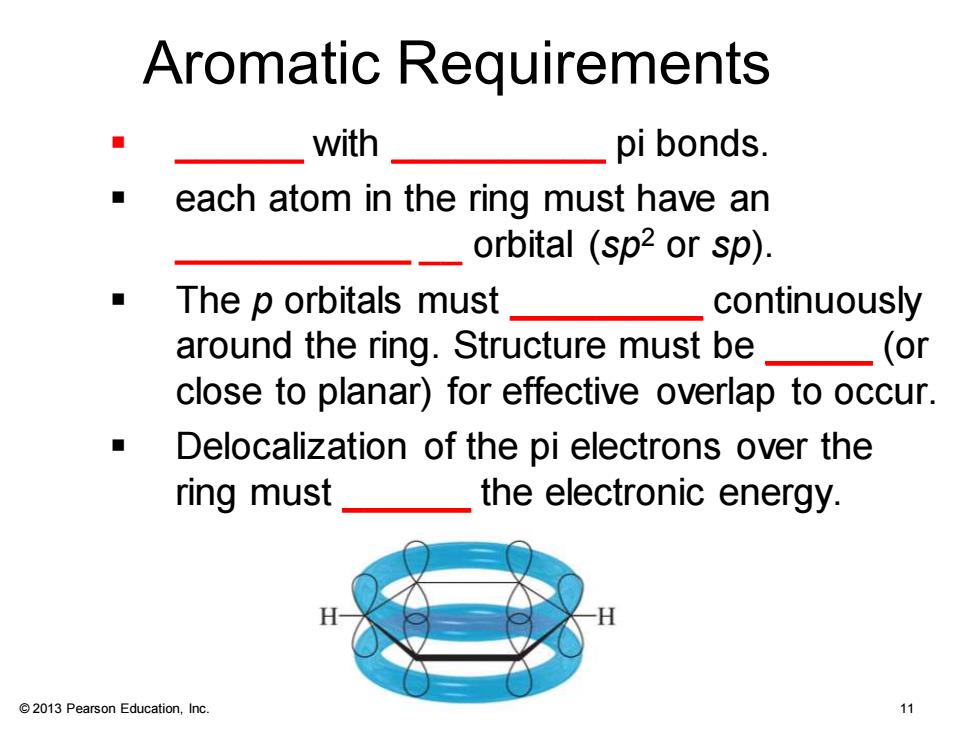
Aromatic Requirements with pi bonds. ◆ each atom in the ring must have an orbital (sp2 or sp). The p orbitals must continuously around the ring.Structure must be (or close to planar)for effective overlap to occur. Delocalization of the pi electrons over the ring must the electronic energy. 2013 Pearson Education,Inc. 11
© 2013 Pearson Education, Inc. Chapter 16 11 Aromatic Requirements ▪ ______ with __________ pi bonds. ▪ each atom in the ring must have an ___________ __ orbital (sp2 or sp). ▪ The p orbitals must _________ continuously around the ring. Structure must be _____ (or close to planar) for effective overlap to occur. ▪ Delocalization of the pi electrons over the ring must ______ the electronic energy
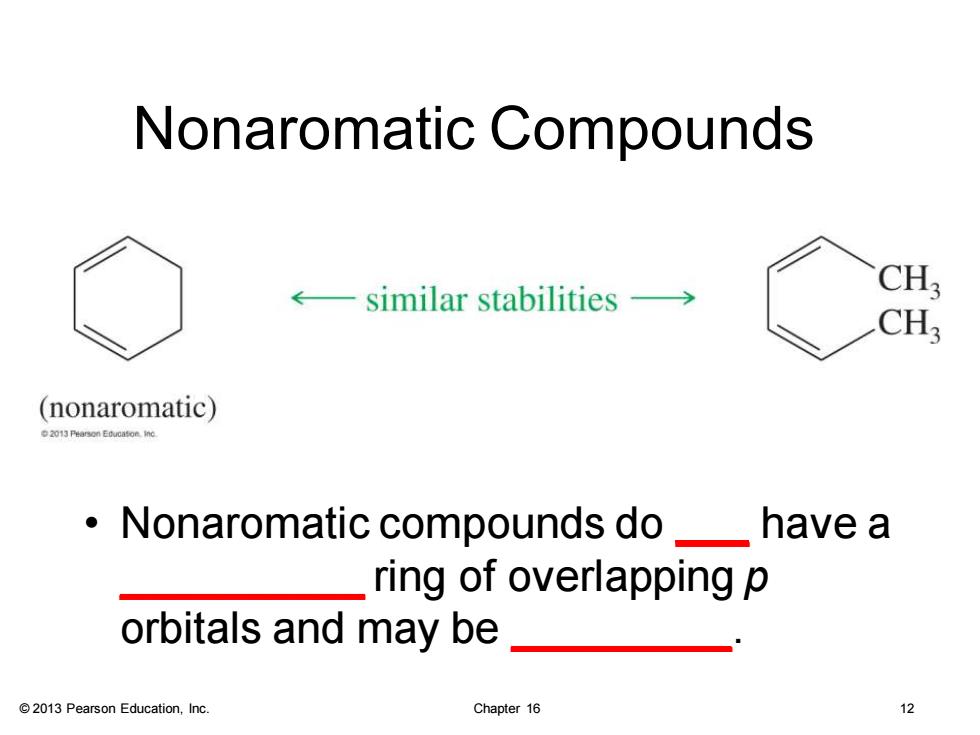
Nonaromatic Compounds similar stabilities-> CH3 CH; (nonaromatic) Nonaromatic compounds do have a ring of overlapping p orbitals and may be 2013 Pearson Education,Inc. Chapter 16
© 2013 Pearson Education, Inc. Chapter 16 12 Nonaromatic Compounds • Nonaromatic compounds do ___ have a __________ ring of overlapping p orbitals and may be _________

Antiaromatic Compounds less stable (antiaromatic) more stable Antiaromatic compounds are and with orbitals around the ring,but electron delocalization its electronic energy. 2013 Pearson Education,Inc. Chapter 16 13
© 2013 Pearson Education, Inc. Chapter 16 13 Antiaromatic Compounds • Antiaromatic compounds are ______ and _________ with _________ p orbitals around the ring, but electron delocalization __________ its electronic energy

Huckel's Rule After the aromatic criteria are met, Huckel's rule applies: ·If the number of pi electrons is(), the system is aromatic (where N is an integer). If the number of pi electrons is ()the system is antiaromatic. 2013 Pearson Education,Inc. Chapter 16
© 2013 Pearson Education, Inc. Chapter 16 14 Hückel’s Rule • After the aromatic criteria are met, Hückel’s rule applies: • If the number of pi electrons is (_____), the system is aromatic (where N is an integer). • If the number of pi electrons is (__), the system is antiaromatic