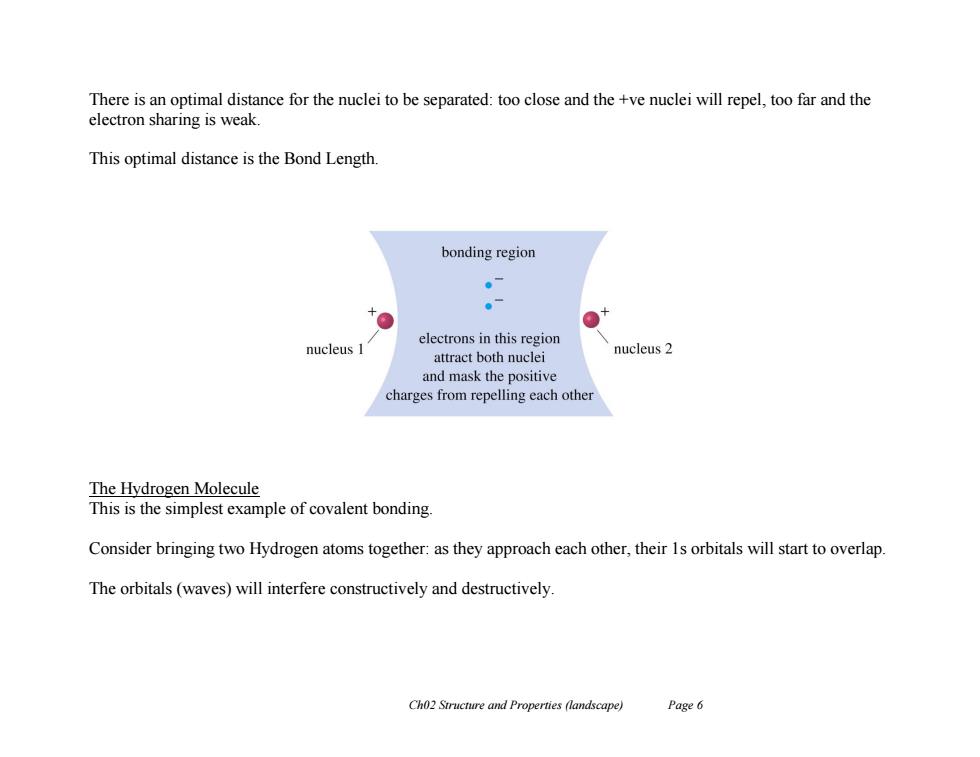
There is an optimal distance for the nuclei to be separated:too close and the +ve nuclei will repel,too far and the electron sharing is weak. This optimal distance is the Bond Length bonding region 。7 。 electrons in this region nucleus 1 attract both nuclei nucleus 2 and mask the positive charges from repelling each other The Hydrogen Molecule This is the simplest example of covalent bonding. Consider bringing two Hydrogen atoms together:as they approach each other,their 1s orbitals will start to overlap. The orbitals(waves)will interfere constructively and destructively Ch02 Structure and Properties (landscape) Page 6
Ch02 Structure and Properties (landscape) Page 6 There is an optimal distance for the nuclei to be separated: too close and the +ve nuclei will repel, too far and the electron sharing is weak. This optimal distance is the Bond Length. The Hydrogen Molecule This is the simplest example of covalent bonding. Consider bringing two Hydrogen atoms together: as they approach each other, their 1s orbitals will start to overlap. The orbitals (waves) will interfere constructively and destructively
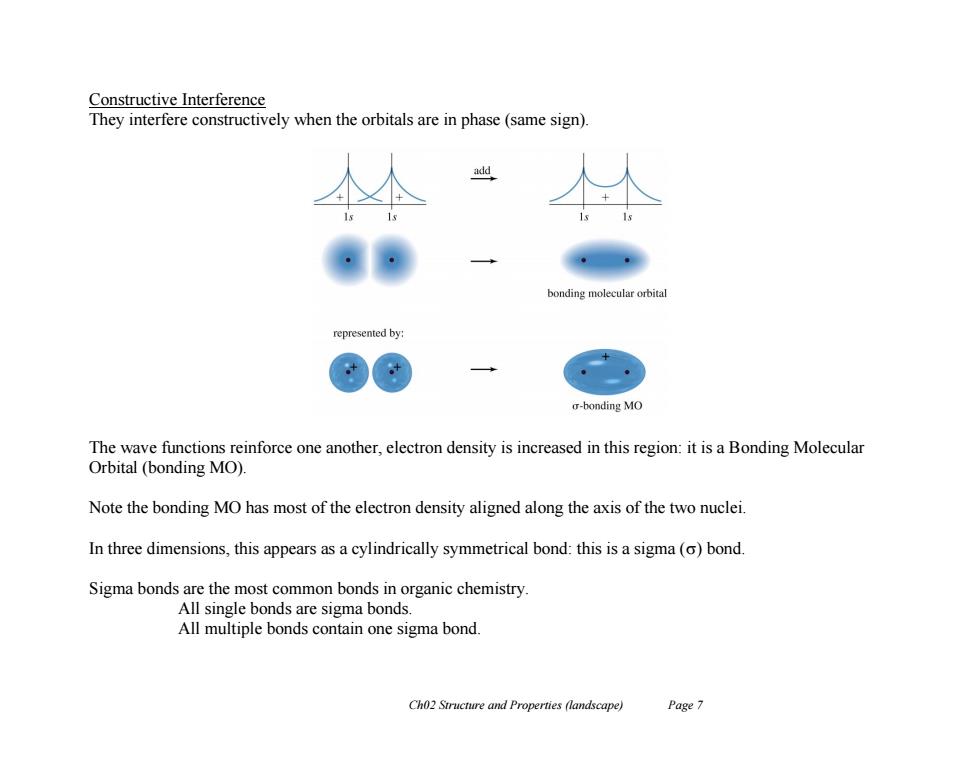
Constructive Interference They interfere constructively when the orbitals are in phase(same sign). add bonding molecular orbital represented by: o-bonding MO The wave functions reinforce one another,electron density is increased in this region:it is a Bonding Molecular Orbital (bonding MO) Note the bonding MO has most of the electron density aligned along the axis of the two nuclei. In three dimensions,this appears as a cylindrically symmetrical bond:this is a sigma(o)bond. Sigma bonds are the most common bonds in organic chemistry. All single bonds are sigma bonds. All multiple bonds contain one sigma bond. Ch02 Structure and Properties (landscape) Page 7
Ch02 Structure and Properties (landscape) Page 7 Constructive Interference They interfere constructively when the orbitals are in phase (same sign). The wave functions reinforce one another, electron density is increased in this region: it is a Bonding Molecular Orbital (bonding MO). Note the bonding MO has most of the electron density aligned along the axis of the two nuclei. In three dimensions, this appears as a cylindrically symmetrical bond: this is a sigma () bond. Sigma bonds are the most common bonds in organic chemistry. All single bonds are sigma bonds. All multiple bonds contain one sigma bond
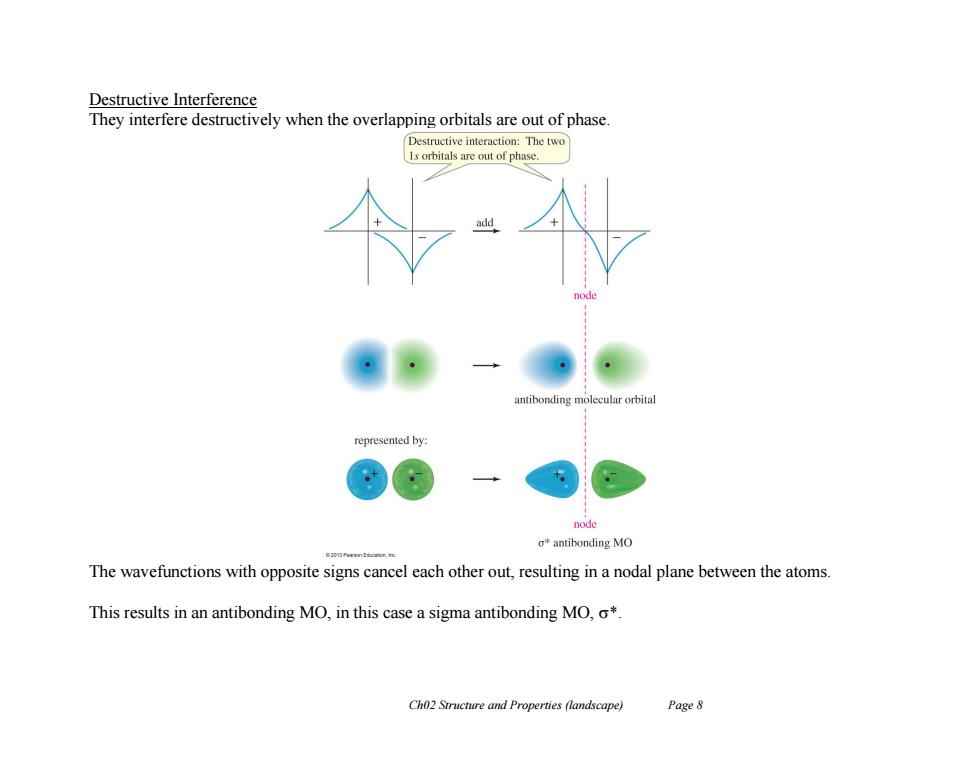
Destructive Interference They interfere destructively when the overlapping orbitals are out of phase. Destructive interaction:The two 1s orbitals are out of phase. antibonding molecular orbita represented by: node o*antibonding MO The wavefunctions with opposite signs cancel each other out,resulting in a nodal plane between the atoms. This results in an antibonding MO,in this case a sigma antibonding MO,o*. Ch02 Structure and Properties (landscape) Page 8
Ch02 Structure and Properties (landscape) Page 8 Destructive Interference They interfere destructively when the overlapping orbitals are out of phase. The wavefunctions with opposite signs cancel each other out, resulting in a nodal plane between the atoms. This results in an antibonding MO, in this case a sigma antibonding MO, *
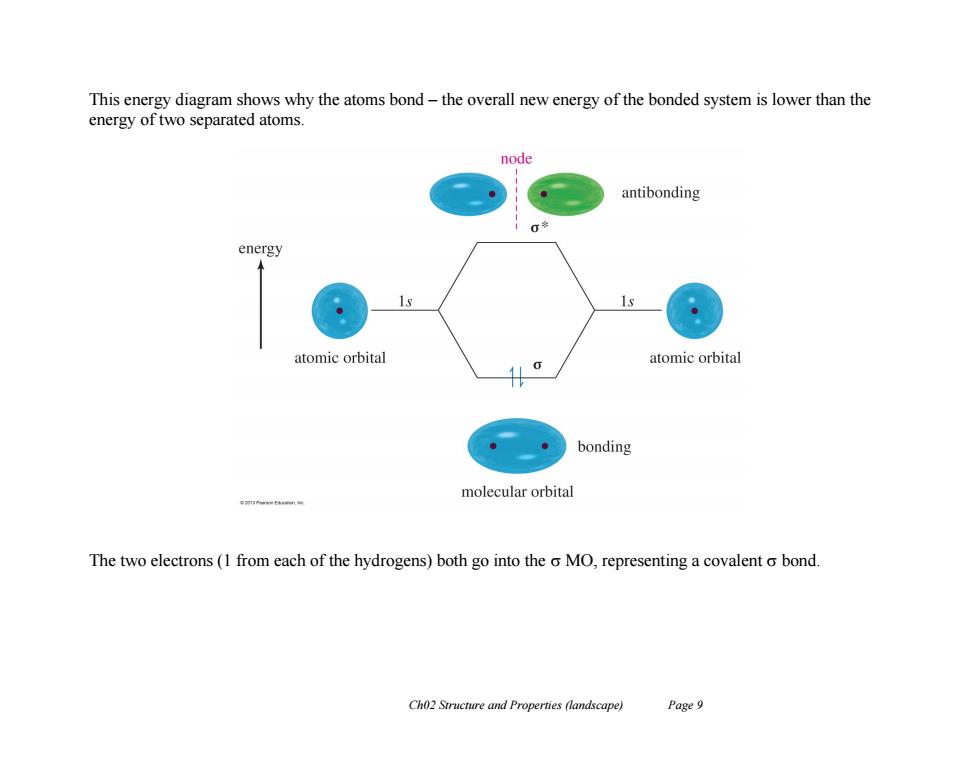
This energy diagram shows why the atoms bond-the overall new energy of the bonded system is lower than the energy of two separated atoms. node antibonding 00 energy 1s atomic orbital atomic orbital bonding molecular orbital The two electrons(1 from each of the hydrogens)both go into the o MO,representing a covalent o bond. Ch02 Structure and Properties (landscape) Page 9
Ch02 Structure and Properties (landscape) Page 9 This energy diagram shows why the atoms bond – the overall new energy of the bonded system is lower than the energy of two separated atoms. The two electrons (1 from each of the hydrogens) both go into the MO, representing a covalent bond
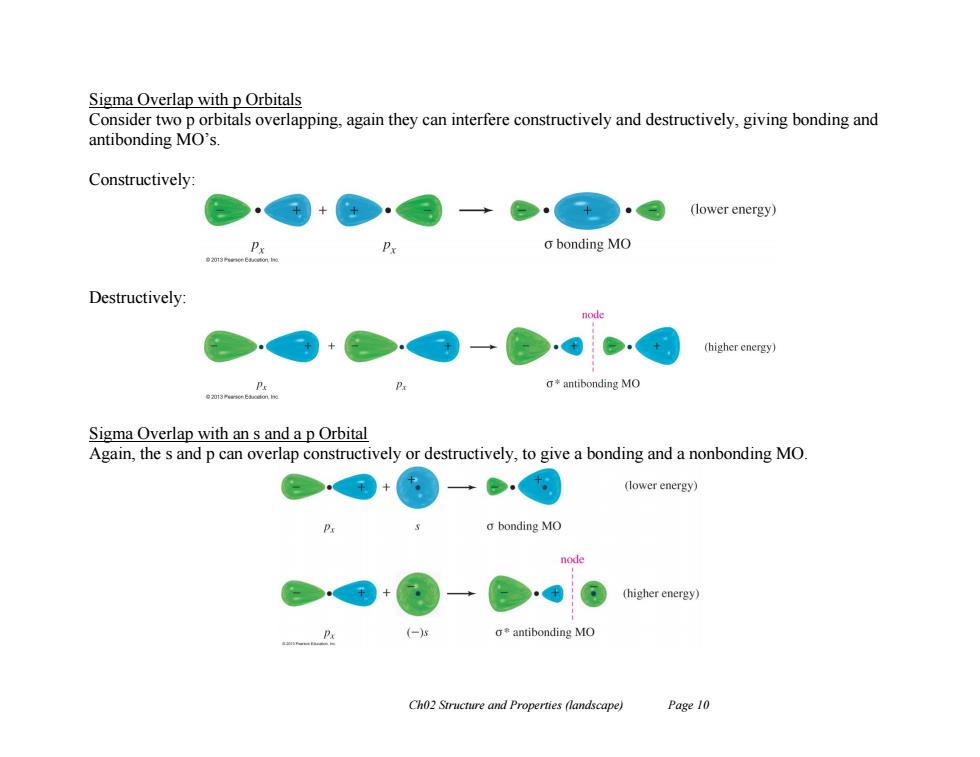
Sigma Overlap with p Orbitals Consider two p orbitals overlapping,again they can interfere constructively and destructively,giving bonding and antibonding MO's Constructively: (lower energy) o bonding MO Destructively: node (higher energy) g*antibonding MO Sigma Overlap with an s and a p Orbital Again,the s and p can overlap constructively or destructively,to give a bonding and a nonbonding MO + (lower energy) o bonding MO node (higher energy) (-) a*antibonding MO Ch02 Structure and Properties (landscape) Page 10
Ch02 Structure and Properties (landscape) Page 10 Sigma Overlap with p Orbitals Consider two p orbitals overlapping, again they can interfere constructively and destructively, giving bonding and antibonding MO’s. Constructively: Destructively: Sigma Overlap with an s and a p Orbital Again, the s and p can overlap constructively or destructively, to give a bonding and a nonbonding MO