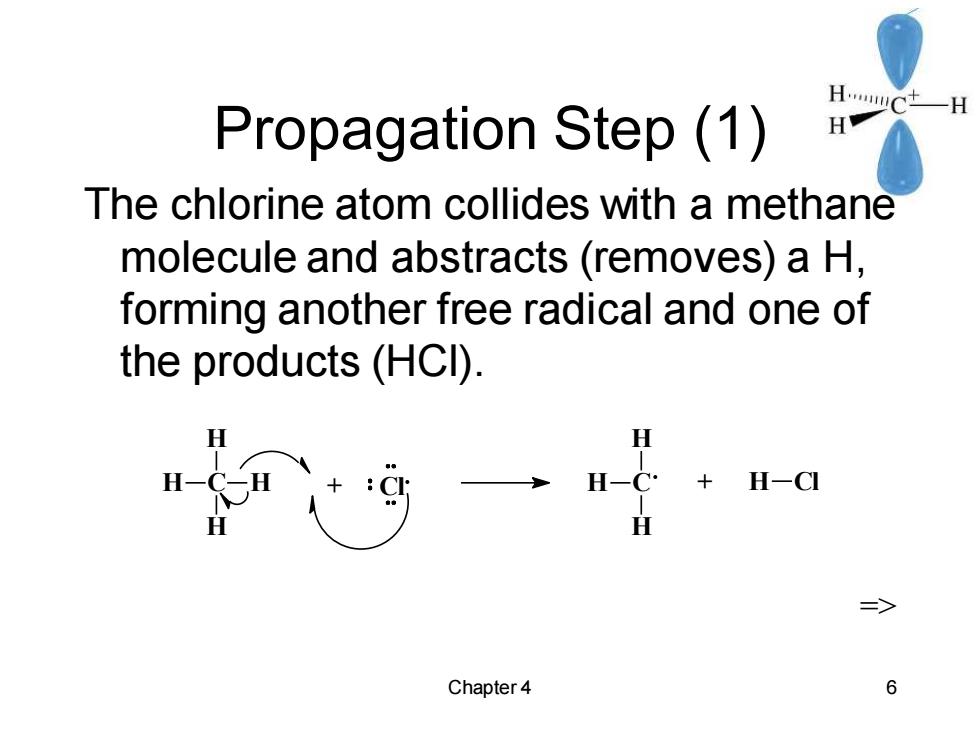
HCtH Propagation Step (1) H The chlorine atom collides with a methane molecule and abstracts (removes)a H, forming another free radical and one of the products(HCI). H H-C H-CI => Chapter 4 6
Chapter 4 6 Propagation Step (1) The chlorine atom collides with a methane molecule and abstracts (removes) a H, forming another free radical and one of the products (HCl). C H H H H + Cl C H H H + H Cl =>
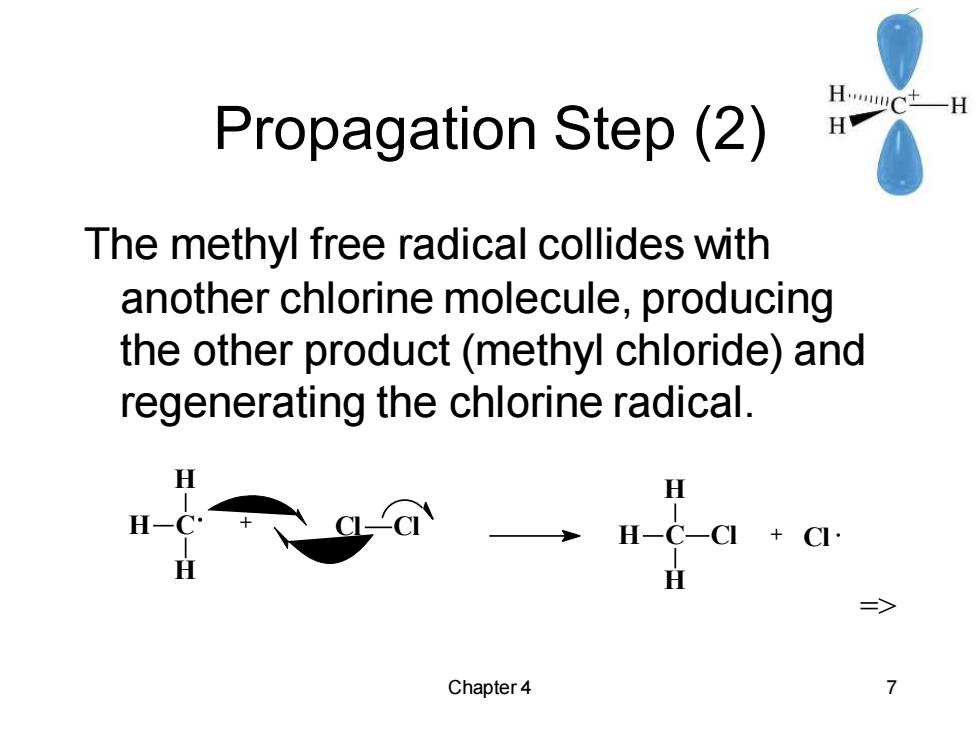
HC -H Propagation Step(2) H The methyl free radical collides with another chlorine molecule,producing the other product(methyl chloride)and regenerating the chlorine radical. H H-C-CI CI H 三> Chapter 4
Chapter 4 7 Propagation Step (2) The methyl free radical collides with another chlorine molecule, producing the other product (methyl chloride) and regenerating the chlorine radical. C H H H + Cl Cl C H H H Cl + Cl =>
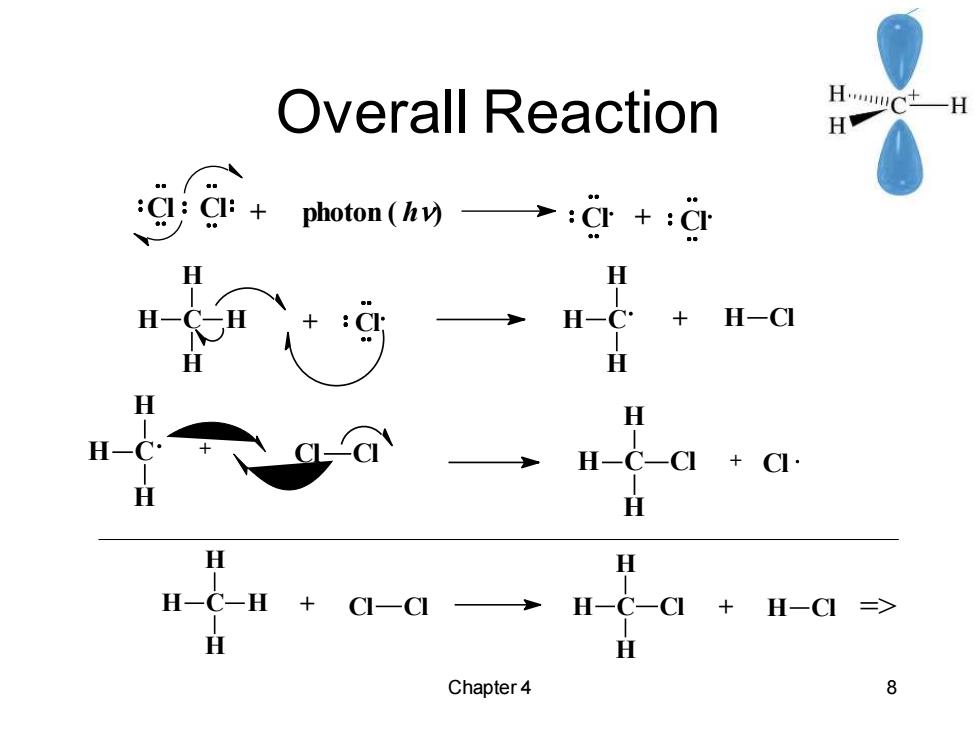
Overall Reaction H.muc -H H :CI:Cl:+photon (v):CI+CI +H-CI H H-C H H -H+C-CI H- +H-C => H Chapter 4 8
Chapter 4 8 Overall Reaction C H H H H + Cl C H H H + H Cl C H H H + Cl Cl C H H H Cl + Cl C H H H H + Cl Cl C H H H Cl + H Cl => Cl Cl + photon ( h) Cl + Cl
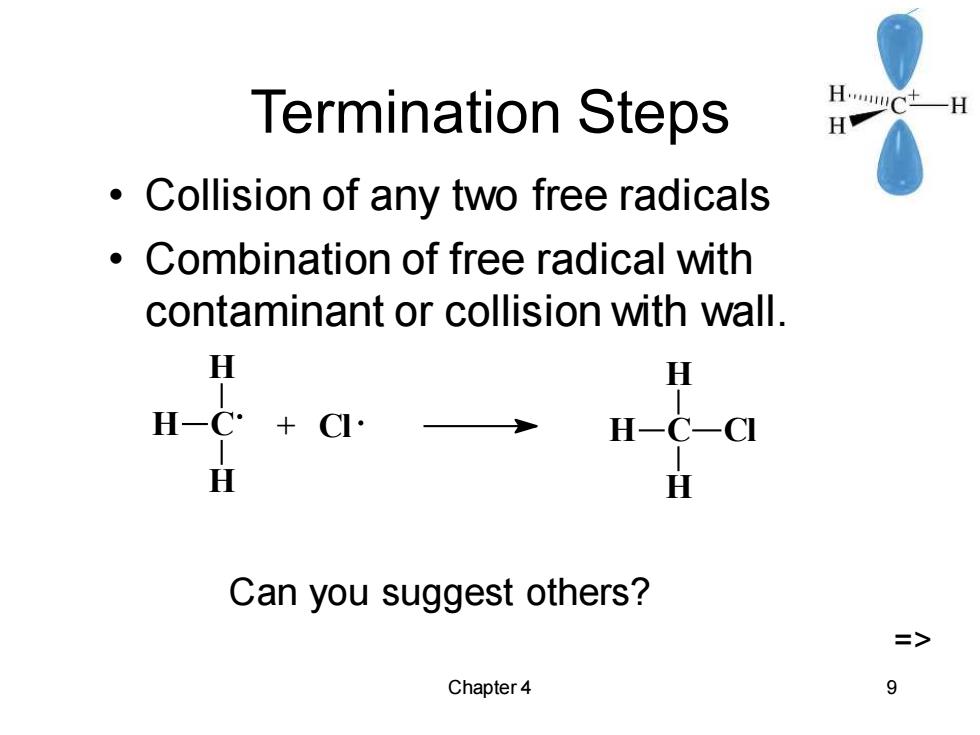
Termination Steps HwC+一H H Collision of any two free radicals 。 Combination of free radical with contaminant or collision with wall. H H H一C: +CI- H-C一C H H Can you suggest others? > Chapter 4 9
Chapter 4 9 Termination Steps • Collision of any two free radicals • Combination of free radical with contaminant or collision with wall. C H H H + Cl C H H H Cl Can you suggest others? =>
HCtH Equilibrium constant H ·Keg=[products] [reactants] For chlorination Keg 1.1 x 1019 ·Large value indicates reaction“goes to completion. => Chapter4 10
Chapter 4 10 Equilibrium constant • Keq = [products] [reactants] • For chlorination Keq = 1.1 x 1019 • Large value indicates reaction “goes to completion.” =>