
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 19 Amines Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 19 Amines Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr

Introduction Organic derivatives of ammonia. Many are biologically active. CH2一CH-COOH OH :NH2 HO CH2 HO CH-CH2-N CH2 CH3 HO HN HO H dopamine epinephrine L-tryptophan a neurotransmitter an adrenal hormone an amino acid 0 CH2OH CH2CH2NH2 HO CH2OH OH H一) H:C > piperazine nicotinic acid pyridoxine histamine kills intestinal worms niacin,a vitamin vitamin B6 dilates blood vessels Copyright 2005 Pearson Prentice Hall,Inc
Chapter 19 2 Introduction • Organic derivatives of ammonia. • Many are biologically active. =>

Biological Activity Neurotransmitters:dopamine Bioregulators:epinephrine ·Vitamins:niacin,.Bs Alkaloids:nicotine,morphine,cocaine 。Amino acids > Chapter 19 3
Chapter 19 3 Biological Activity • Neurotransmitters: dopamine • Bioregulators: epinephrine • Vitamins: niacin, B6 • Alkaloids: nicotine, morphine, cocaine • Amino acids =>
Classes of Amines Primary (1):one C-N bond,2 N-H bonds. 。 Secondary (2):two C-N bonds,1 N-H bond. Tertiary (3):three C-N bonds,no N-H bond. Quaternary (4):four C-N bonds,nitrogen has a formal charge. => Chapter 19 4
Chapter 19 4 Classes of Amines • Primary (1): one C-N bond, 2 N-H bonds. • Secondary (2): two C-N bonds, 1 N-H bond. • Tertiary (3): three C-N bonds, no N-H bond. • Quaternary (4): four C-N bonds, nitrogen has a + formal charge. =>
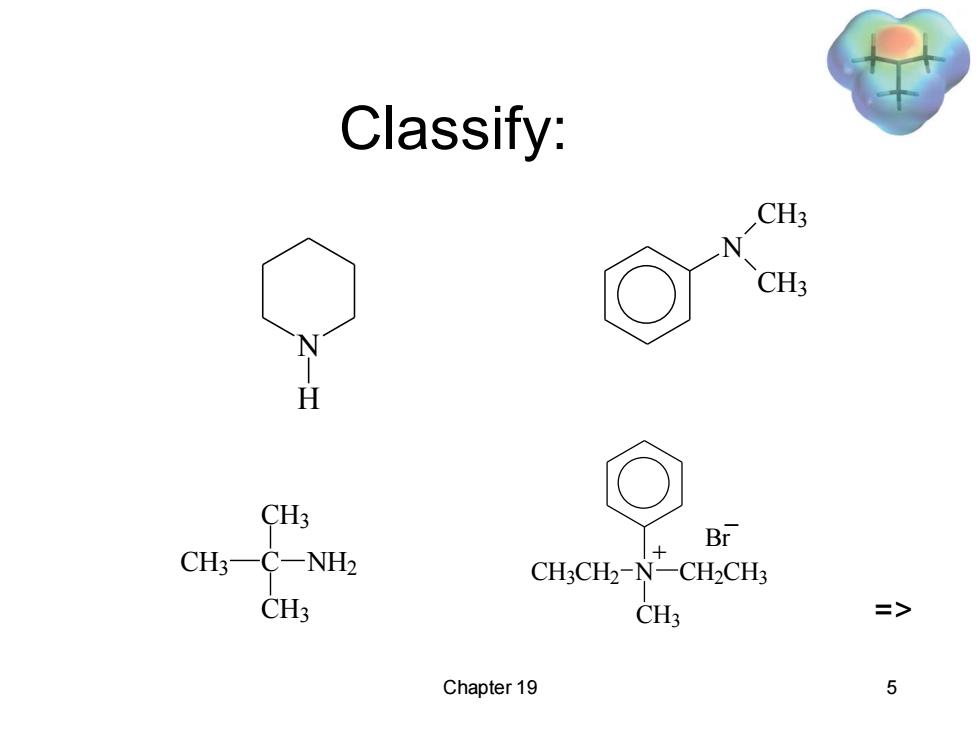
Classify: CH3 CH3 CH3 Br CH3 C-NH2 CH3CH2-N-CH2CH3 CH3 CH3 三> Chapter 19 5
Chapter 19 5 Classify: N H CH3 C CH3 CH3 NH2 N CH3 CH3 CH3CH2 N CH3 CH2CH3 + Br _ =>