
Organic Chemistry,5th Edition L.G.Wade,Jr. Alkenes:Chapter 8 Reactions of Alkenes
Alkenes: Chapter 8 Reactions of Alkenes Organic Chemistry, 5th Edition L. G. Wade, Jr
Reactivity of C=C 111 Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. Carbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the double bond. => Chapter 8 2
Chapter 8 2 Reactivity of C=C • Electrons in pi bond are loosely held. • Electrophiles are attracted to the pi electrons. • Carbocation intermediate forms. • Nucleophile adds to the carbocation. • Net result is addition to the double bond. =>
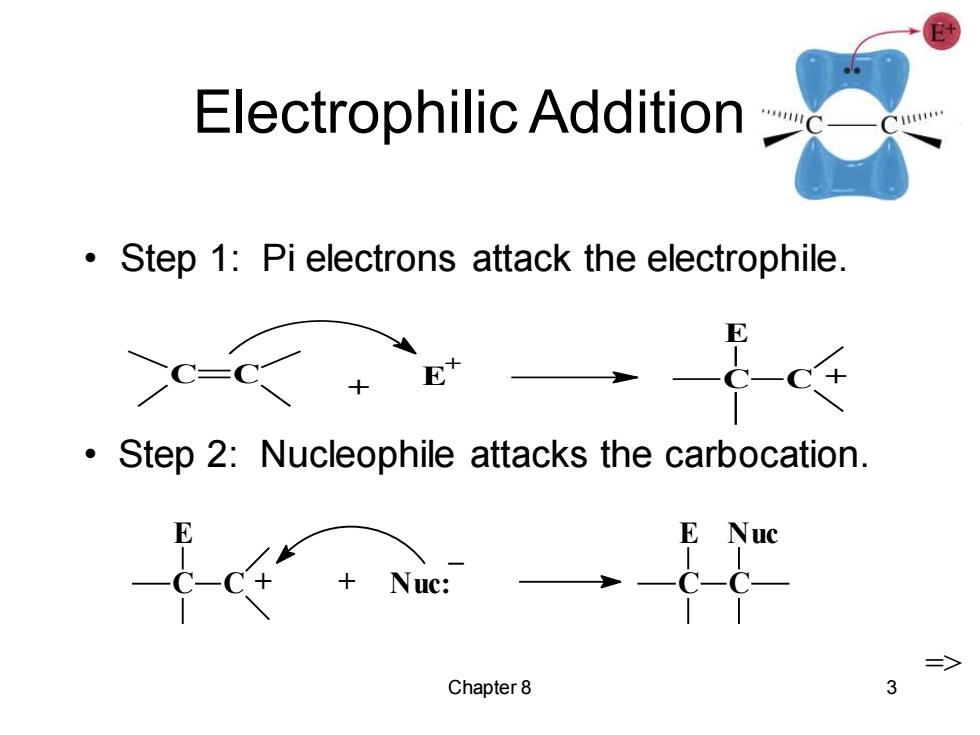
Electrophilic Addition Step 1:Pi electrons attack the electrophile. Step 2:Nucleophile attacks the carbocation. E Nuc C-C- => Chapter8 3
Chapter 8 3 Electrophilic Addition • Step 1: Pi electrons attack the electrophile. C C + E + C E C + C E C + + Nuc: _ C E C Nuc => • Step 2: Nucleophile attacks the carbocation
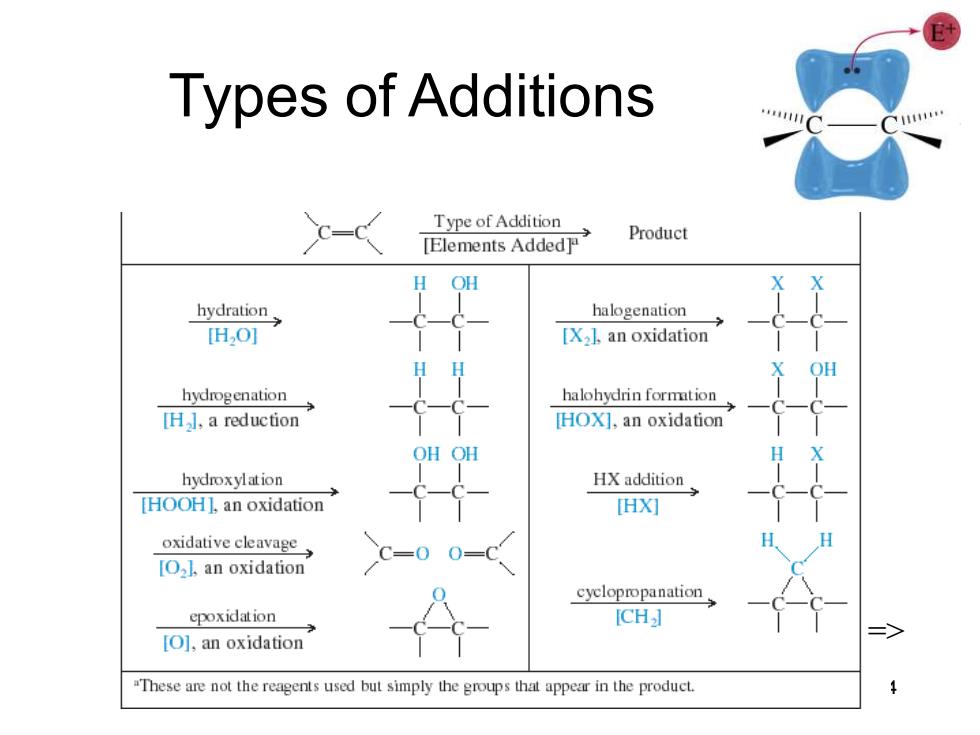
Types of Additions 111 Type of Addition [Elements Added Product H OH hydration halogenation [H2O] [X2l.an oxidation OH hydrogenation halohydrin formtion [H],a reduction [HOX],an oxidation OH OH hydroxylation HX addition [HOOH],an oxidation HX灯 oxidative cavage C=0 [O2l.an oxidation cyclopropanation epoxidation [CH] [O],an oxidation "These are not the reagents used but simply the groups that appear in the product
Chapter 8 4 Types of Additions =>
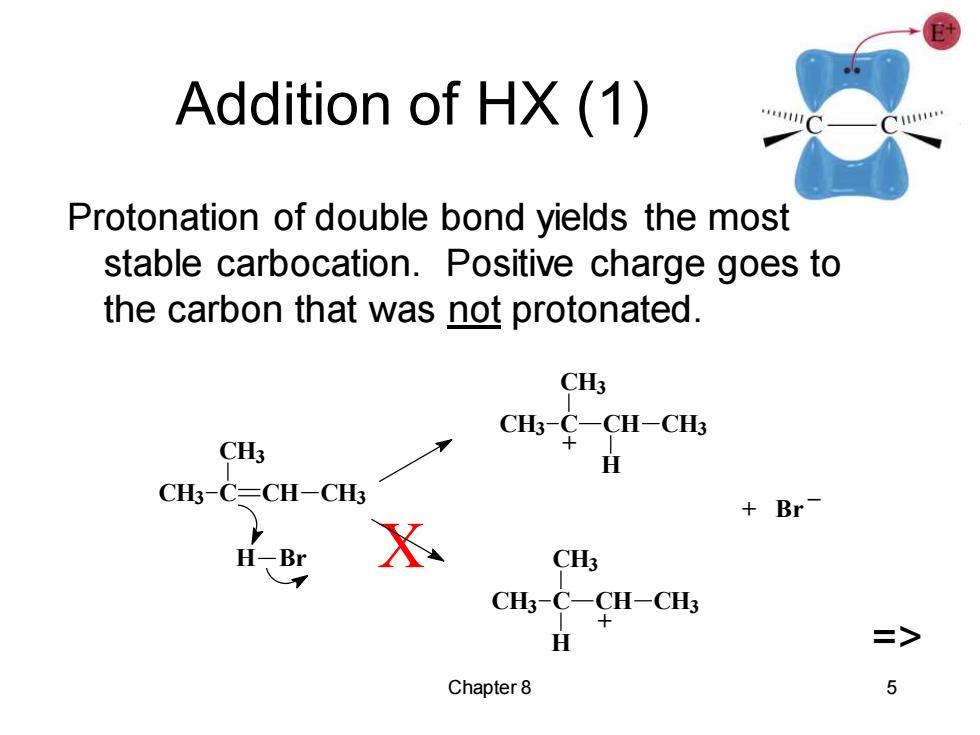
Addition of HX(1) Protonation of double bond yields the most stable carbocation.Positive charge goes to the carbon that was not protonated. CH3 CH3 CHs¥CH-C4 H CH3-C=CH-CH3 Br H-Br X CH3 CH3-C-CH-CH3 H => Chapter 8 5
Chapter 8 5 Addition of HX (1) Protonation of double bond yields the most stable carbocation. Positive charge goes to the carbon that was not protonated. X => + Br _ + + CH3 C CH3 CH CH3 H CH3 C CH3 CH CH3 H H Br CH3 C CH3 CH CH3