
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 5 Stereochemistry Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 5 Stereochemistry Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall Organic Chemistry, 6th Edition L. G. Wade, Jr

Stereoisomers Same bonding sequence. Different arrangement in space. Example:HOOC-CH=CH-COOH has two geometric(cis-trans)isomers: 0 O H &-0H o C-OH C=0 C=C HO-C H H H fumaric acid,mp 287C maleic acid,mp 138 C essential metabolite Chapter5 toxic irritant 2
Chapter 5 2 Stereoisomers • Same bonding sequence. • Different arrangement in space. • Example: HOOC-CH=CH-COOH has two geometric (cis-trans) isomers: HO C O C H C H C O OH fumaric acid, mp 287 C essential metabolite o H C C C H C O OH O HO maleic acid, mp 138 C toxic irritant o =>

Chirality ·“Handedness”:right glove doesn'tfit the left hand. 。 Mirror-image object is different from the original object. => right hand left hand Copyright 2005 Pearson Prentice Hall,Inc. 3
Chapter 5 3 Chirality • “Handedness”: right glove doesn’t fit the left hand. • Mirror-image object is different from the original object. =>
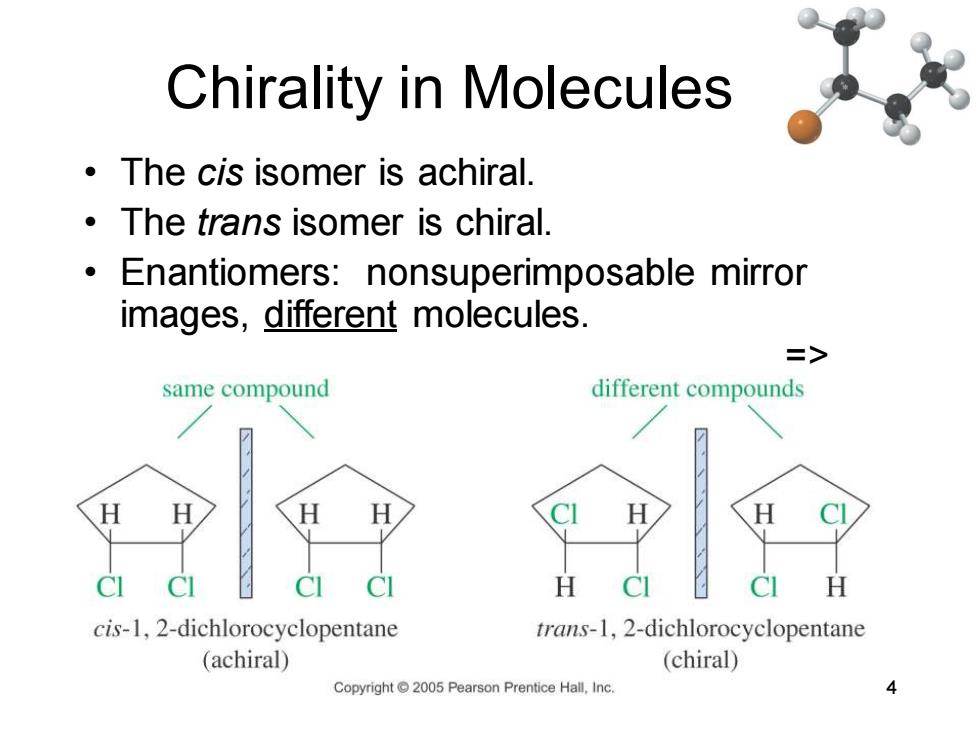
Chirality in Molecules The cis isomer is achiral. The trans isomer is chiral. Enantiomers:nonsuperimposable mirror images,different molecules. => same compound different compounds H cis-1,2-dichlorocyclopentane trans-1,2-dichlorocyclopentane (achiral) (chiral) Copyright2005 Pearson Prentice Hall,Inc. 4
Chapter 5 4 Chirality in Molecules • The cis isomer is achiral. • The trans isomer is chiral. • Enantiomers: nonsuperimposable mirror images, different molecules. =>

Stereocenters Any atom at which the exchange of two groups yields a stereoisomer. ·Examples: ·Asymmetric carbons Double-bonded carbons in cis-trans isomers CH2CH3 CH2CH2CH3 CH3 CH2CH3 H3 Br CH(CH3)2 asymmetric carbon chirality centers ( stereocenters(circled) Copyright2005 Pearson Prentice Hall,Inc
Chapter 5 5 Stereocenters • Any atom at which the exchange of two groups yields a stereoisomer. • Examples: • Asymmetric carbons • Double-bonded carbons in cis-trans isomers =>