
Alkenes:Reactions and Synthesis Based on McMurry's Organic Chemistry,6th edition
Alkenes: Reactions and Synthesis Based on McMurry’s Organic Chemistry, 6th edition
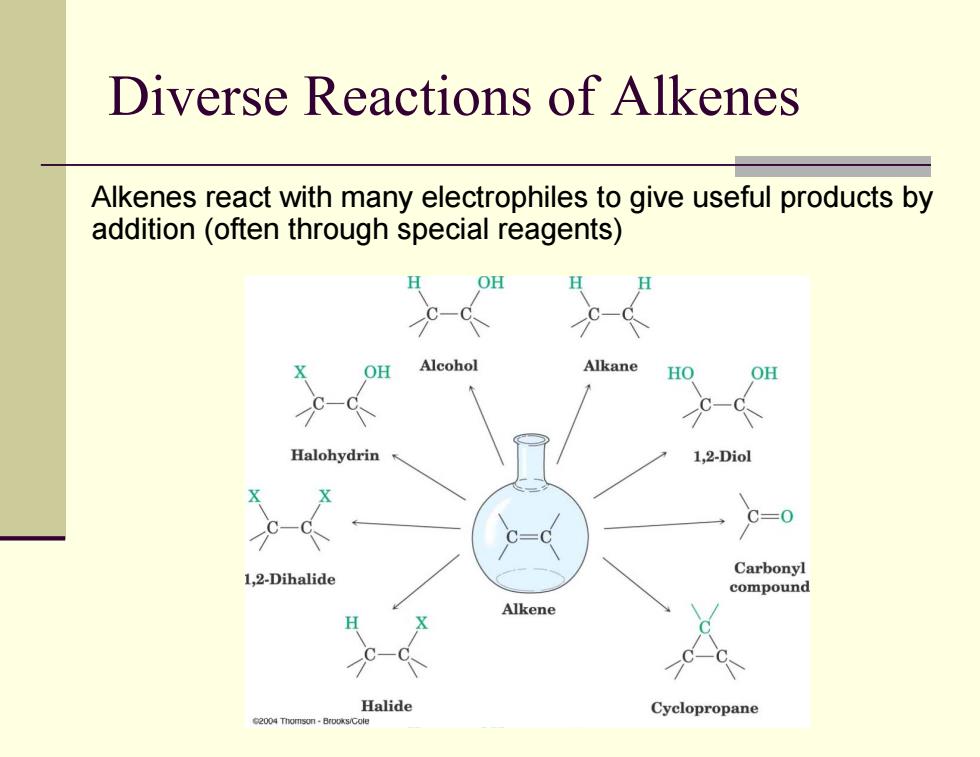
Diverse Reactions of alkenes Alkenes react with many electrophiles to give useful products by addition (often through special reagents) OH OH Alcohol Alkane HO OH Halohydrin 1,2-Diol C=0 1,2-Dihalide Carbonyl compound Alkene C. Halide Cyclopropane 2004 Thomson-Brooks/Gol
Diverse Reactions of Alkenes Alkenes react with many electrophiles to give useful products by addition (often through special reagents)
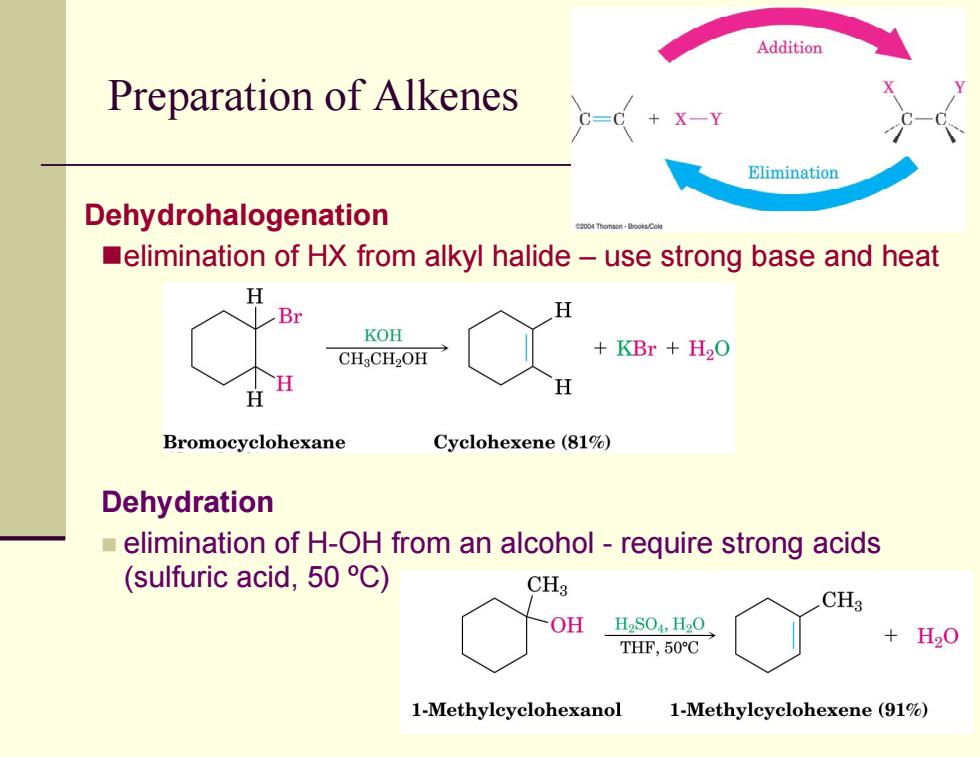
Addition Preparation of Alkenes G=0 X-Y Elimination Dehydrohalogenation elimination of HX from alkyl halide-use strong base and heat H H KOH CH3CH2OH KBr H2O H Bromocyclohexane Cyclohexene(81%) Dehydration elimination of H-OH from an alcohol-require strong acids (sulfuric acid,50 C) CH3 CHs OH HS04,H20 THF,50C +H20 1-Methylcyclohexanol 1-Methylcyclohexene (91%)
Preparation of Alkenes Dehydrohalogenation elimination of HX from alkyl halide – use strong base and heat Dehydration elimination of H-OH from an alcohol - require strong acids (sulfuric acid, 50 ºC)

Addition of Halogens to Alkenes ■ Bromine and chlorine add to alkenes to give 1,2-dihalides F2 is too reactive and 12 does not add Cl2 reacts as Cl+Cl,Br2 behaves similarly Br H H Br—Br Br H Br Br Cyclopentene trans-1,2-Dibromo- cis-1,2-Dibromo- cyclopentane cyclopentane (Sole product) (Not formed) 2004 Thomson-Brooks/Cole Reaction occurs with anti stereochemistry What is the mechanism?
Addition of Halogens to Alkenes Bromine and chlorine add to alkenes to give 1,2-dihalides F 2 is too reactive and I 2 does not add Cl 2 reacts as Cl + Cl- , Br 2 behaves similarly Reaction occurs with anti stereochemistry What is the mechanism?
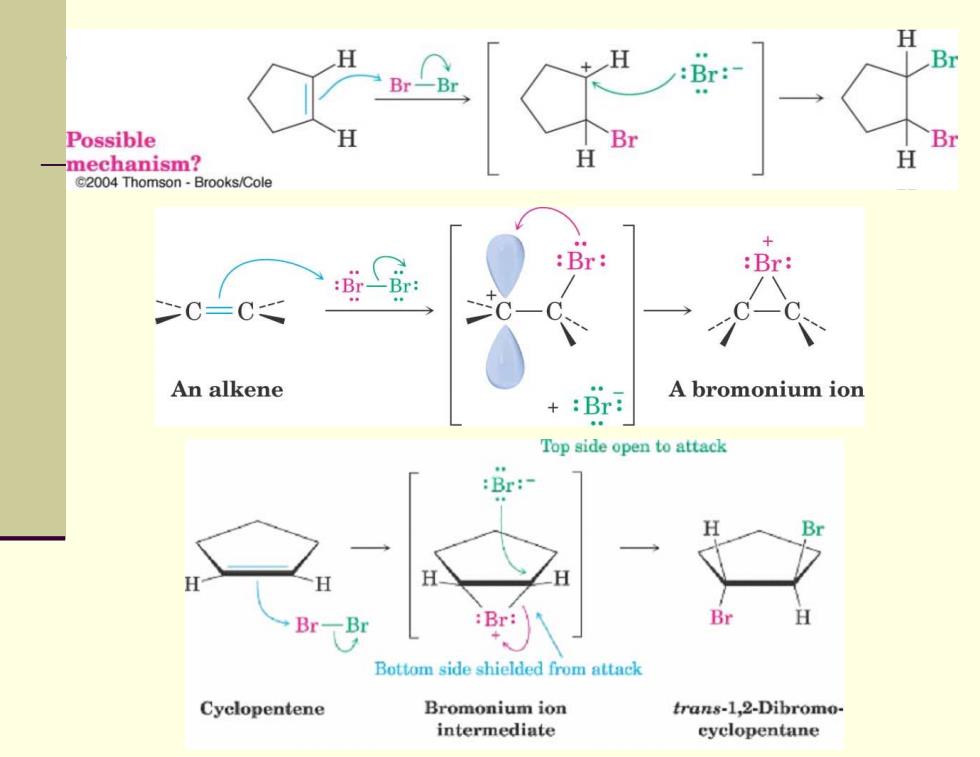
H Br-Br Br: Possible B mechanism? H @2004 Thomson-Brooks/Cole + :Br: :Br: C=C An alkene A bromonium ion +:Br: Top side open to attack :Br:- H Br H Br-Br :Br: Br Bottom side shielded from attack Cyclopentene Bromonium ion trans-1,2-Dibromo- intermediate cyclopentane