
Alkynes Based on McMurry's Organic Chemistry,6th edition,Chapter 8
Alkynes Based on McMurry’s Organic Chemistry, 6th edition, Chapter 8
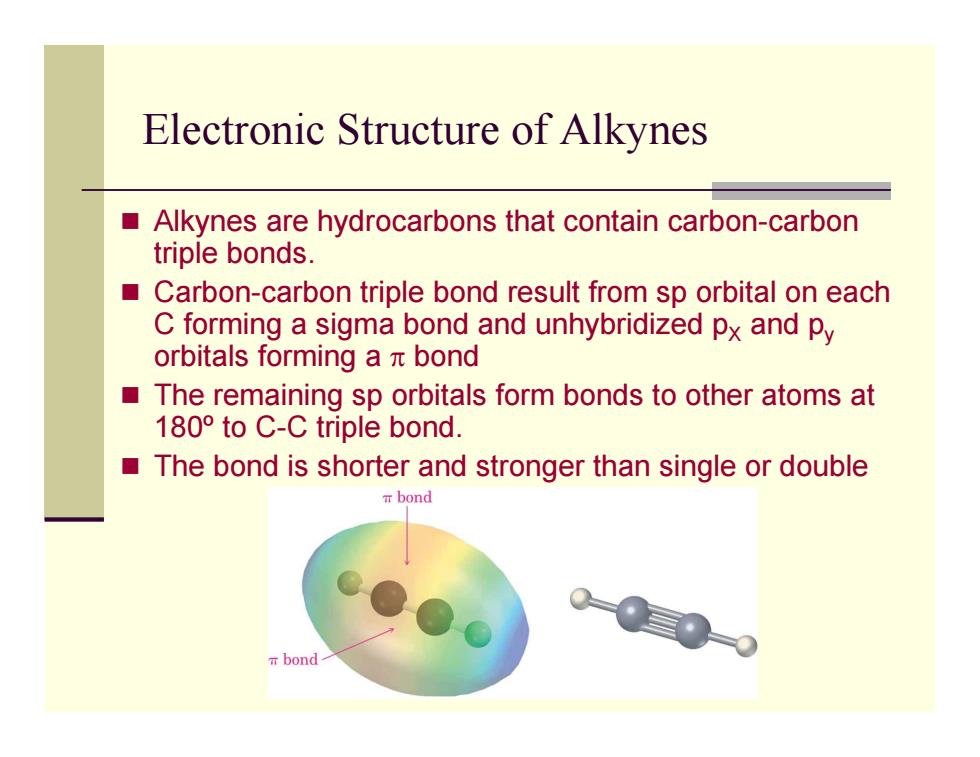
Electronic Structure of Alkynes Alkynes are hydrocarbons that contain carbon-carbon triple bonds. ■ Carbon-carbon triple bond result from sp orbital on each C forming a sigma bond and unhybridized px and py orbitals forming a t bond The remaining sp orbitals form bonds to other atoms at 180 to C-C triple bond. The bond is shorter and stronger than single or double T bond m bond
Electronic Structure of Alkynes Alkynes are hydrocarbons that contain carbon-carbon triple bonds. Carbon-carbon triple bond result from sp orbital on each C forming a sigma bond and unhybridized p X and p y orbitals forming a π bond The remaining sp orbitals form bonds to other atoms at 180º to C-C triple bond. The bond is shorter and stronger than single or double
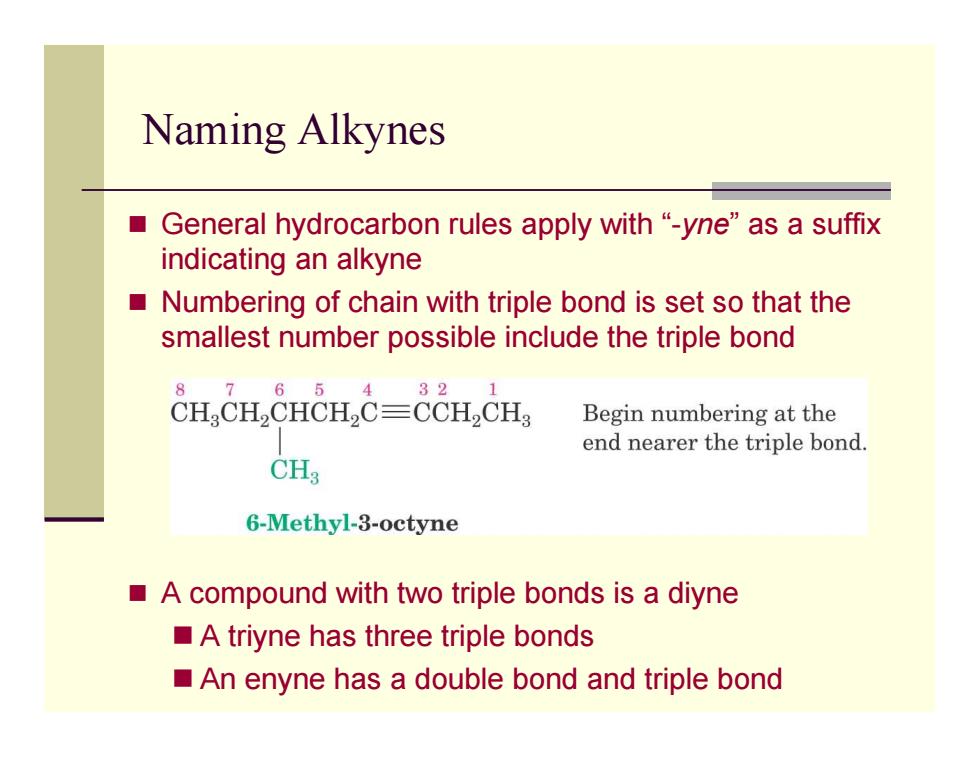
Naming Alkynes ■ General hydrocarbon rules apply with "-yne"as a suffix indicating an alkyne ■ Numbering of chain with triple bond is set so that the smallest number possible include the triple bond 8 7 65 432 CH CHCHCH2C=CCH2CH Begin numbering at the end nearer the triple bond. CHg 6-Methyl-3-octyne A compound with two triple bonds is a diyne A triyne has three triple bonds An enyne has a double bond and triple bond
Naming Alkynes General hydrocarbon rules apply with “-yne” as a suffix indicating an alkyne Numbering of chain with triple bond is set so that the smallest number possible include the triple bond A compound with two triple bonds is a diyne A triyne has three triple bonds An enyne has a double bond and triple bond
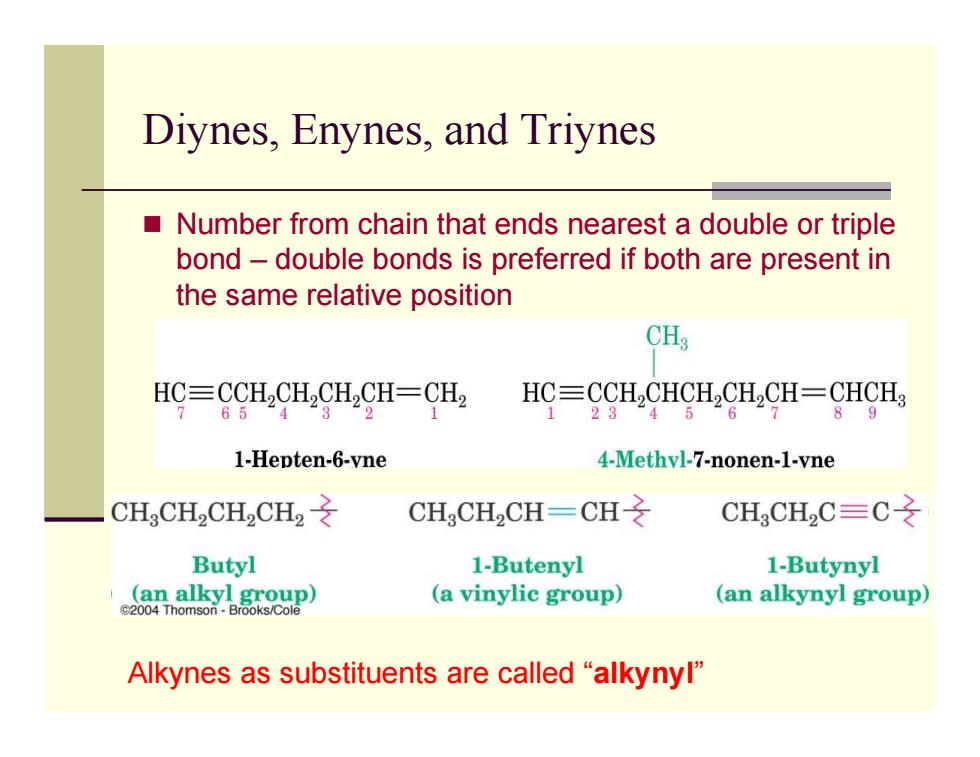
Diynes,Enynes,and Triynes Number from chain that ends nearest a double or triple bond-double bonds is preferred if both are present in the same relative position CH3 HC三CCH2CHCH2CH=CH2 HC=CCH,CHCH2CH2CH=CHCH3 65 3 2 1 23 45 89 1-Hepten-6-vne 4-Methvl-7-nonen-1-vne CHCH2CH2CH2之 CH CH2CH-CH CH3CH2C=C之 Butyl 1-Butenyl 1-Butynyl 4aal黑e (a vinylic group) (an alkynyl group) Alkynes as substituents are called "alkynyl
Diynes, Enynes, and Triynes Number from chain that ends nearest a double or triple bond – double bonds is preferred if both are present in the same relative position Alkynes as substituents are called “alkynyl
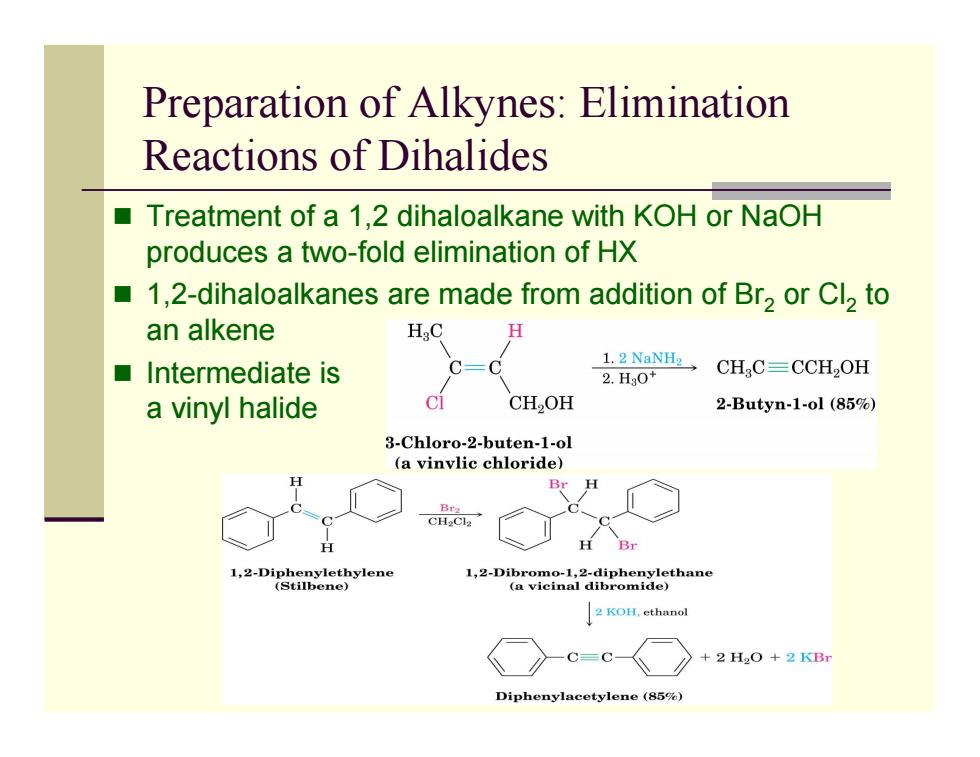
Preparation of Alkynes:Elimination Reactions of Dihalides ■ Treatment of a 1,2 dihaloalkane with KOH or NaOH produces a two-fold elimination of HX ■ 1,2-dihaloalkanes are made from addition of Br2 or Cl2 to an alkene HC ■Intermediate is 1.2 NaNH2 2.Hg0+ CHC=CCH,OH a vinyl halide C CH2OH 2-Butyn-1-ol (85%) 3-Chloro-2-buten-1-ol (a vinvlic chloride) Br H B型 1,2-Diphenylethylene (Stilbene) 1-Dticina)thane 2 KOH,ethanol C=C- +2H2O+2B Diphenylacetylene(85%)
Preparation of Alkynes: Elimination Reactions of Dihalides Treatment of a 1,2 dihaloalkane with KOH or NaOH produces a two-fold elimination of HX 1,2-dihaloalkanes are made from addition of Br 2 or Cl 2 to an alkene Intermediate is a vinyl halide