
Organic Chemistry,6th Edition L.G.Wade,Jr. Chapter 1 Introduction and Review Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2006,Prentice Hall
Chapter 1 Introduction and Review Organic Chemistry, 6th Edition L. G. Wade, Jr. Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2006, Prentice Hall

Definitions ·Old:“derived from living organisms” ·New:“chemistry of carbon compounds” From inorganic to organic,Wohler,1828 NH OCN heat、 urea 三> Chapter 1
Chapter 1 2 Definitions • Old: “derived from living organisms” • New: “chemistry of carbon compounds” • From inorganic to organic, Wöhler, 1828 heat NH4 + OCN - H2 N C NH2 O urea =>
Atomic Structure Atoms:protons,neutrons,and electrons. The number of protons determines the identity of the element. Some atoms of the same element have a different number of neutrons.These are called isotopes. Example:12C,13C,and 14C. => Chapter 1 3
Chapter 1 3 Atomic Structure • Atoms: protons, neutrons, and electrons. • The number of protons determines the identity of the element. • Some atoms of the same element have a different number of neutrons. These are called isotopes. • Example: 12C, 13C, and 14C. =>
Electronic Structure Electrons:outside the nucleus,in orbitals. Electrons have wave properties. Electron density is the probability of finding the electron in a particular part of an orbital. 。 Orbitals are grouped into“shells,”at different distances from the nucleus. > Chapter 1 4
Chapter 1 4 Electronic Structure • Electrons: outside the nucleus, in orbitals. • Electrons have wave properties. • Electron density is the probability of finding the electron in a particular part of an orbital. • Orbitals are grouped into “shells,” at different distances from the nucleus. =>
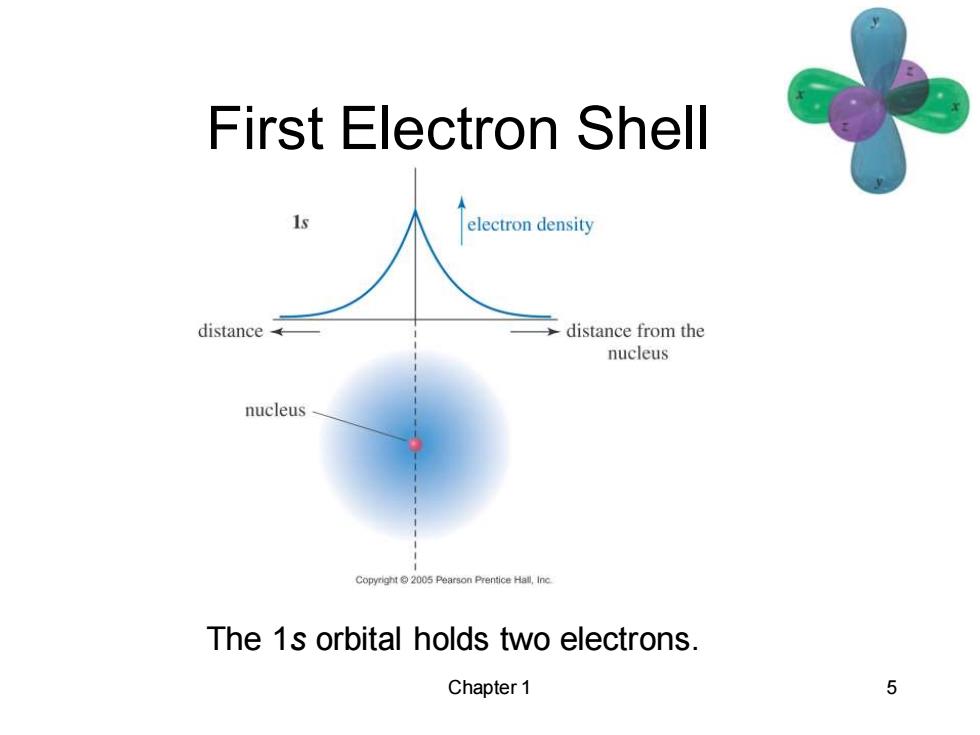
First Electron Shell electron density distance distance from the nucleus nucleus Copyright2005 Pearson Prentice Hall,Inc The 1s orbital holds two electrons. Chapter 1 5
Chapter 1 5 First Electron Shell The 1s orbital holds two electrons