
Stereochemistry of Alkanes and Cycloalkanes Based on McMurry's Organic Chemistry,6th edition,Chapter 4
Stereochemistry of Alkanes and Cycloalkanes Based on McMurry’s Organic Chemistry, 6th edition, Chapter 4
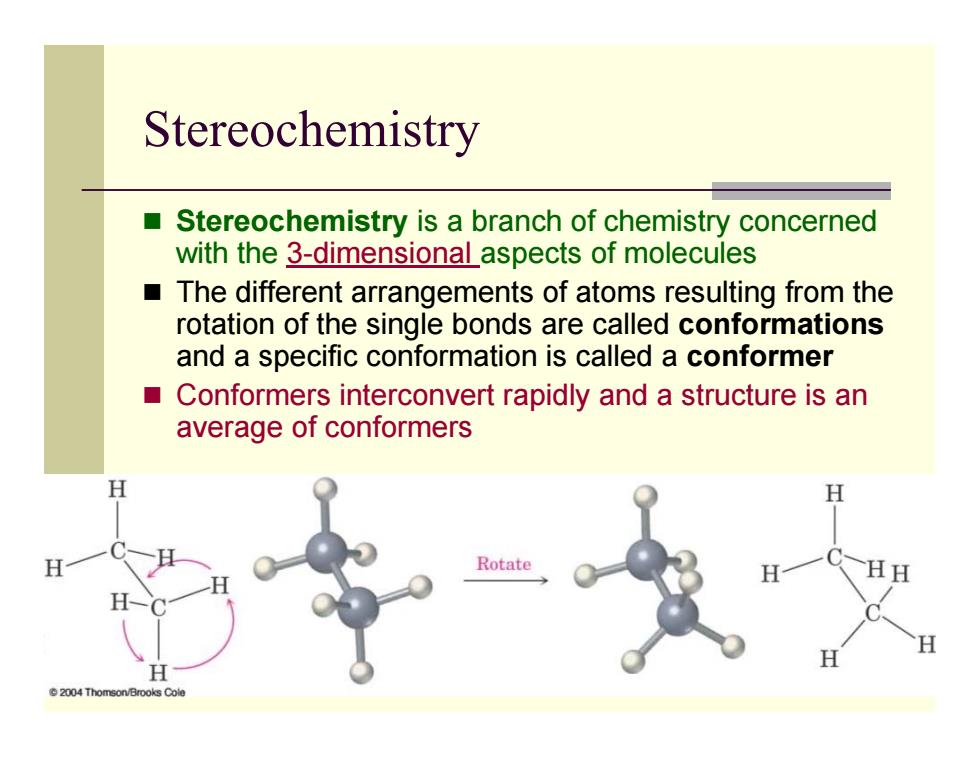
Stereochemistry Stereochemistry is a branch of chemistry concerned with the 3-dimensionaLaspects of molecules The different arrangements of atoms resulting from the rotation of the single bonds are called conformations and a specific conformation is called a conformer Conformers interconvert rapidly and a structure is an average of conformers H H 2004 Thomson/Brooks Cole
Stereochemistry Stereochemistry is a branch of chemistry concerned with the 3-dimensional aspects of molecules The different arrangements of atoms resulting from the rotation of the single bonds are called conformations and a specific conformation is called a conformer Conformers interconvert rapidly and a structure is an average of conformers
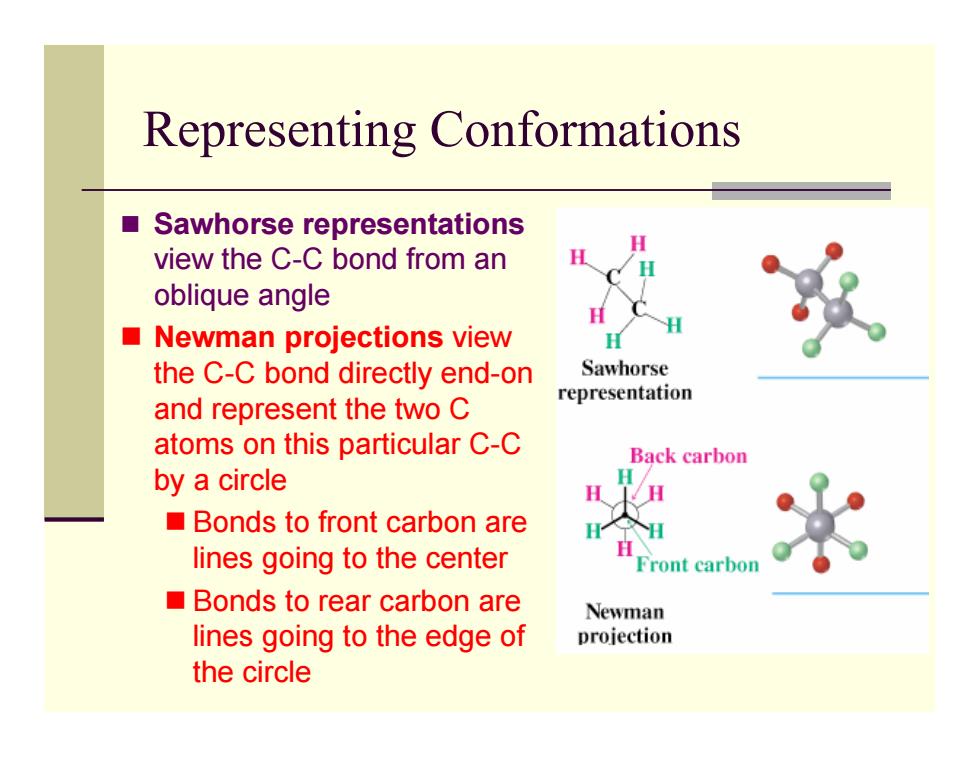
Representing Conformations ■ Sawhorse representations view the C-C bond from an oblique angle Newman projections view the C-C bond directly end-on Sawhorse representation and represent the two C atoms on this particular C-C Back carbon by a circle Bonds to front carbon are lines going to the center Front carbon Bonds to rear carbon are Newman lines going to the edge of projection the circle
Representing Conformations Sawhorse representations view the C-C bond from an oblique angle Newman projections view the C-C bond directly end-on and represent the two C atoms on this particular C-C by a circle Bonds to front carbon are lines going to the center Bonds to rear carbon are lines going to the edge of the circle
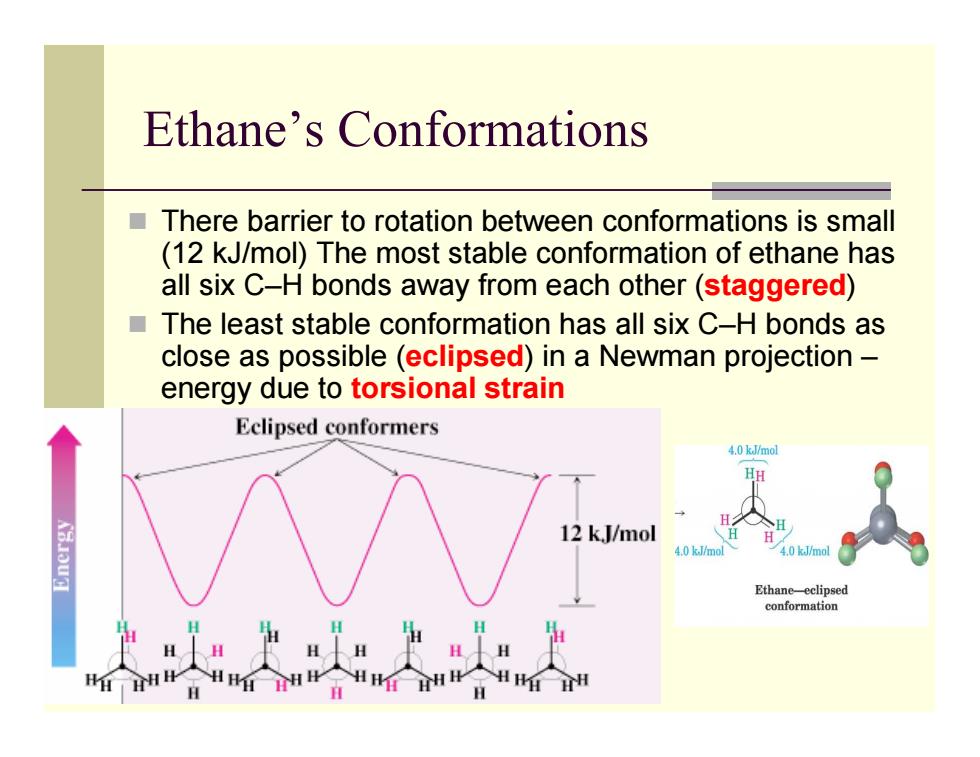
Ethane's Conformations There barrier to rotation between conformations is small (12 kJ/mol)The most stable conformation of ethane has all six C-H bonds away from each other(staggered) The least stable conformation has all six C-H bonds as close as possible (eclipsed)in a Newman projection- energy due to torsional strain Eclipsed conformers 4.0 kJ/mol HH 八 12 k,J/mol 4.0 kJ/mo 4.0 kJ/mol Ethane-eclipsed conformation
Ethane’s Conformations There barrier to rotation between conformations is small (12 kJ/mol) The most stable conformation of ethane has all six C–H bonds away from each other (staggered ) The least stable conformation has all six C–H bonds as close as possible (eclipsed) in a Newman projection – energy due to torsional strain
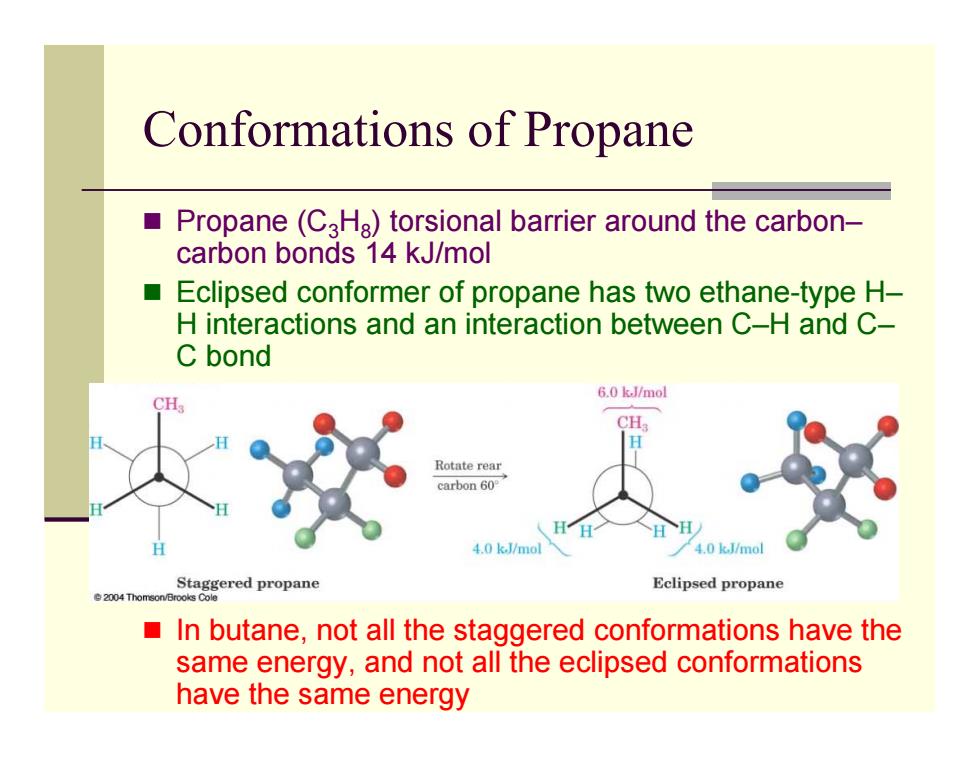
Conformations of Propane Propane(CaHa)torsional barrier around the carbon- carbon bonds 14 kJ/mol ■ Eclipsed conformer of propane has two ethane-type H- H interactions and an interaction between C-H and C- C bond 6.0 kJ/mol CH Rotate rear carbon60° 、HH HH 4.0 k.J/mol 4.0 k.J/mol ered propane Eclipsed propane In butane,not all the staggered conformations have the same energy,and not all the eclipsed conformations have the same energy
Conformations of Propane Propane (C 3 H 8) torsional barrier around the carbon– carbon bonds 14 kJ/mol Eclipsed conformer of propane has two ethane-type H– H interactions and an interaction between C–H and C– C bond In butane, not all the staggered conformations have the same energy, and not all the eclipsed conformations have the same energy