
Polar Covalent Bonds; Acids and Bases Based on McMurry's Organic Chemistry,6th edition,Chapter 2
Polar Covalent Bonds; Acids and Bases Based on McMurry’s Organic Chemistry, 6th edition, Chapter 2
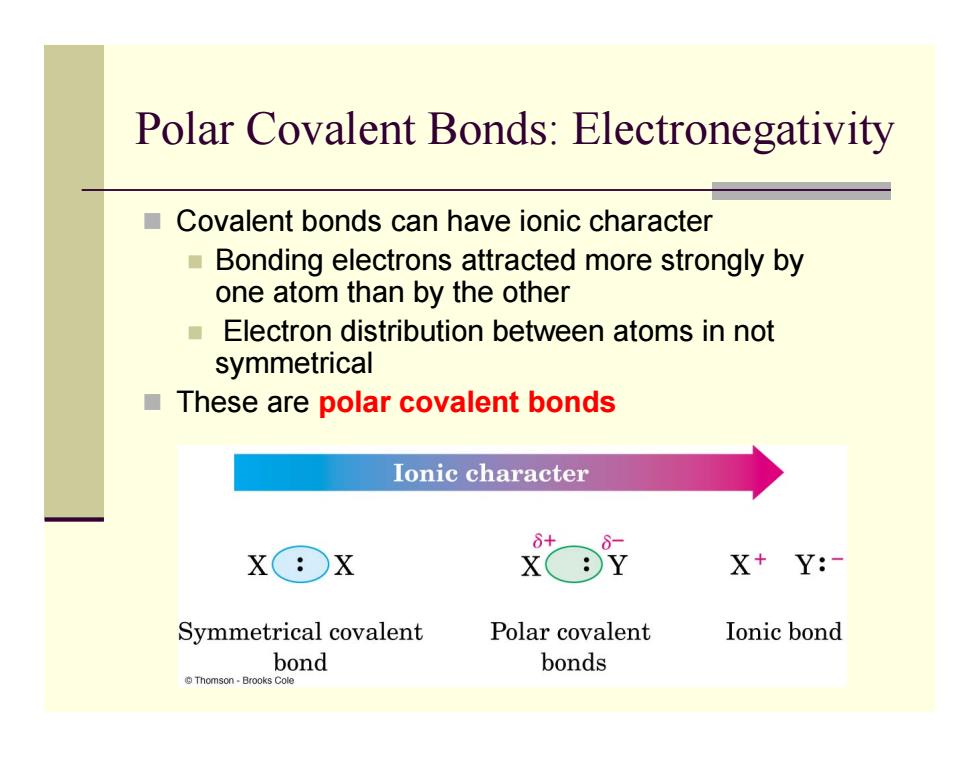
Polar Covalent Bonds:Electronegativity Covalent bonds can have ionic character Bonding electrons attracted more strongly by one atom than by the other ■ Electron distribution between atoms in not symmetrical These are polar covalent bonds Ionic character X:X X+Y:- Symmetrical covalent Polar covalent Ionic bond bond bonds Thomson-Brooks Cole
Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character Bonding electrons attracted more strongly by one atom than by the other Electron distribution between atoms in not symmetrical These are polar covalent bonds
Bond Polarity and Electronegativity ■ Electronegativity (EN):intrinsic ability of an atom to attract the shared electrons in a covalent bond Differences in EN produce bond polarity Metals on left side of periodic table attract electrons weakly,lower EN Halogens and other reactive nonmetals on right side of periodic table attract electrons strongly,higher electronegativities F is most electronegative(EN 4.0),Cs is least(EN =0.7) ■EN of C=2.5
Bond Polarity and Electronegativity Electronegativity (EN): intrinsic ability of an atom to attract the shared electrons in a covalent bond Differences in EN produce bond polarity Metals on left side of periodic table attract electrons weakly, lower EN Halogens and other reactive nonmetals on right side of periodic table attract electrons strongly, higher electronegativities F is most electronegative (EN = 4.0), Cs is least (EN = 0.7) EN of C = 2.5
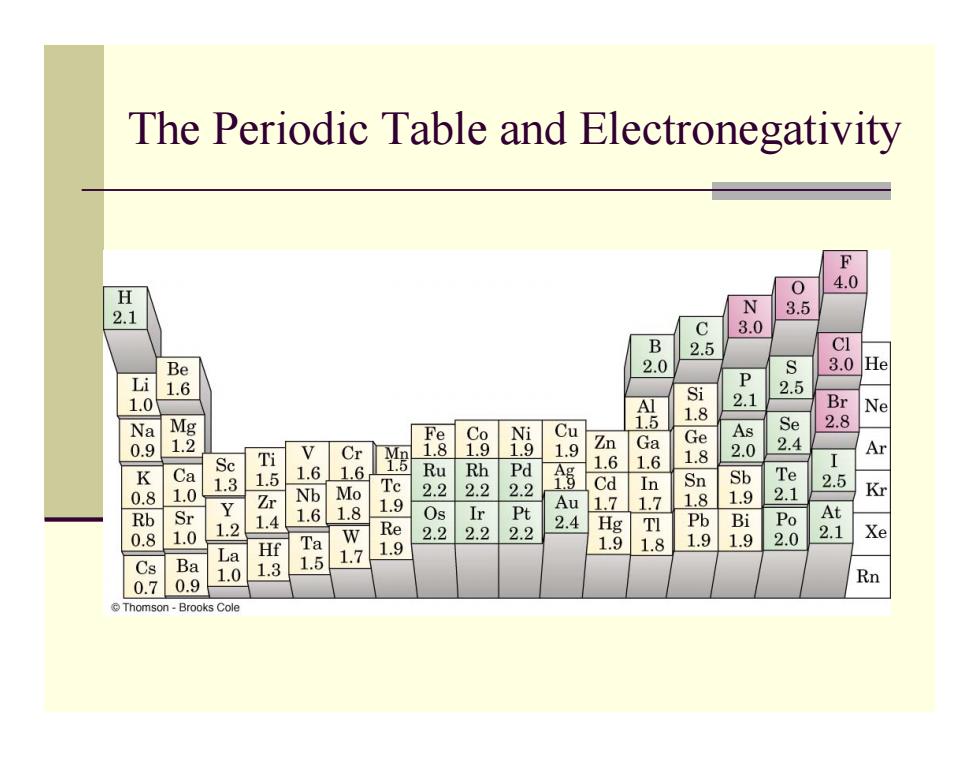
The Periodic Table and Electronegativity F 4.0 H 0 2.1 N . 3.0 B 2.5 CI Be 3.0 8 1.6 P S Mg Si 1.8 0.9 2 V Cr 遇 g a 41059 54 Br 2.8 8 Se Ti 6 I Ar 1.6 1.6 R 1.0 1.3 1.5 Te 2.2 Rh 0.8 Nb Mo 2 Cd Sn 1.9 1 2 .8 组 Kr Rb Sr 1.4 1.6 1.8 1.2 Re 2.2 2.2 2西 Hg I 的 Po At .8 1.0 Ta 1.9 1.8 .0 2.1 La H 1.9 Cs Ba . 1.5 1.7 0.70.9 .0 Rn ⊙Thomson-Brooks Cole
The Periodic Table and Electronegativity
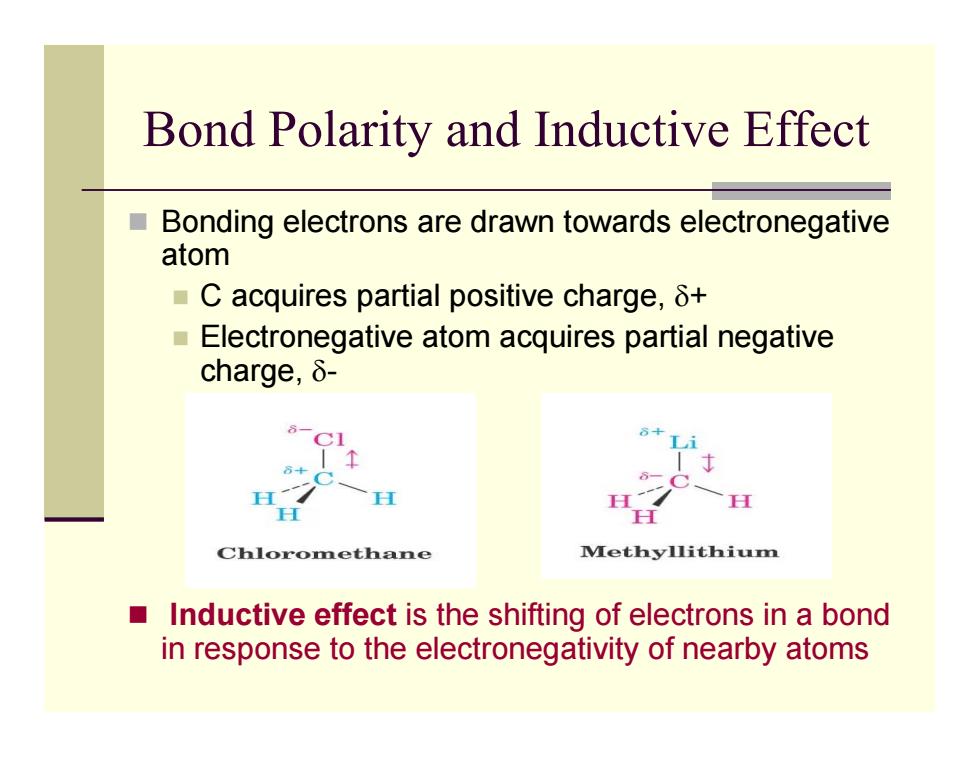
Bond Polarity and Inductive Effect ■ Bonding electrons are drawn towards electronegative atom ■C acquires partial positive charge,δt ■ Electronegative atom acquires partial negative charge,δ- H Chloromethane Methyllithium Inductive effect is the shifting of electrons in a bond in response to the electronegativity of nearby atoms
Bond Polarity and Inductive Effect Bonding electrons are drawn towards electronegative atom C acquires partial positive charge, δ + Electronegative atom acquires partial negative charge, δ - Inductive effect is the shifting of electrons in a bond in response to the electronegativity of nearby atoms