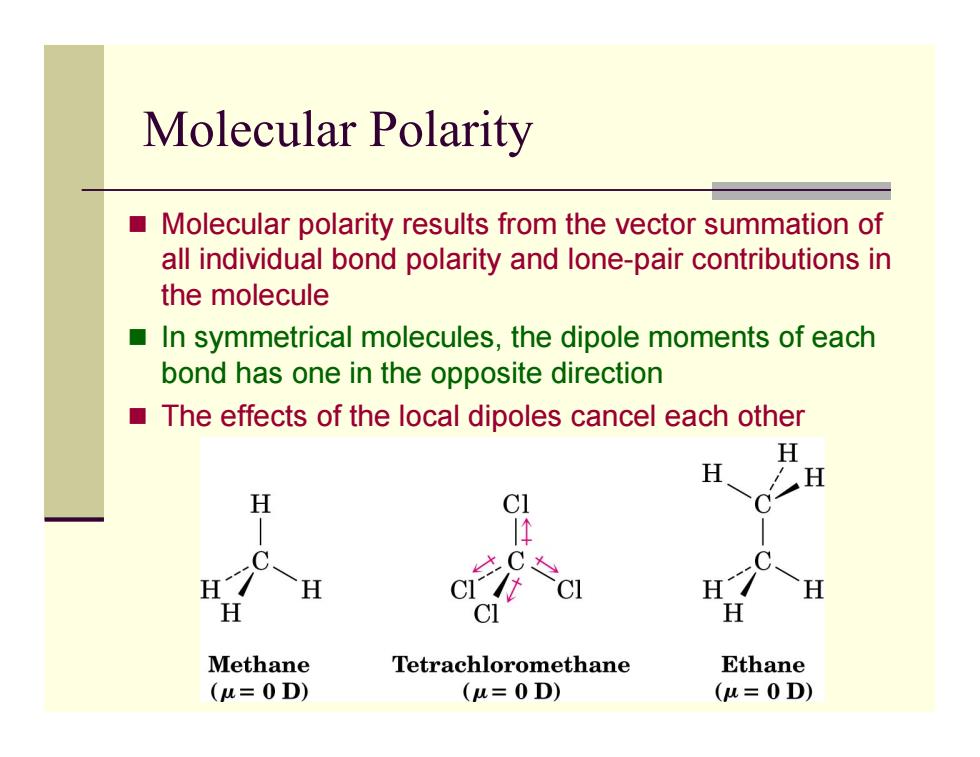
Molecular Polarity Molecular polarity results from the vector summation of all individual bond polarity and lone-pair contributions in the molecule ■ In symmetrical molecules,the dipole moments of each bond has one in the opposite direction The effects of the local dipoles cancel each other H H H H C1 必C& H H H H CI H Methane Tetrachloromethane Ethane (u=0D) (u=0D) (u=0D)
Molecular Polarity Molecular polarity results from the vector summation of all individual bond polarity and lone-pair contributions in the molecule In symmetrical molecules, the dipole moments of each bond has one in the opposite direction The effects of the local dipoles cancel each other
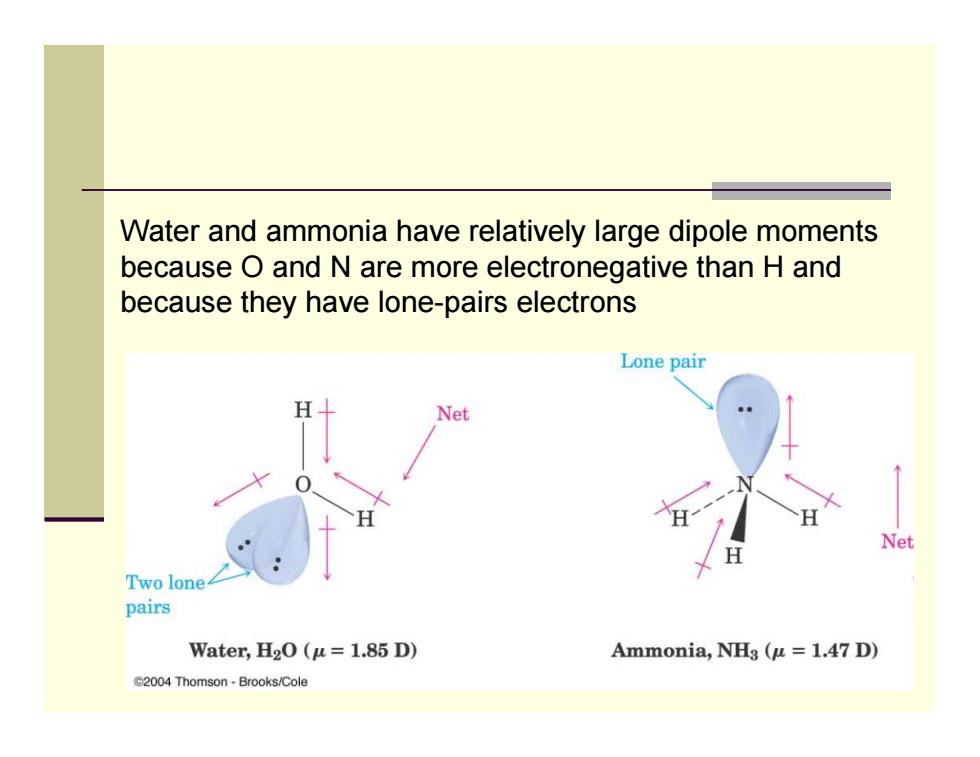
Water and ammonia have relatively large dipole moments because O and N are more electronegative than H and because they have lone-pairs electrons Lone pair Net Net Two lone pairs Water,H2O(u=1.85 D) Ammonia,NHg (u =1.47 D) G2004 Thomson-Brooks/Cole
Water and ammonia have relatively large dipole moments because O and N are more electronegative than H and because they have lone-pairs electrons
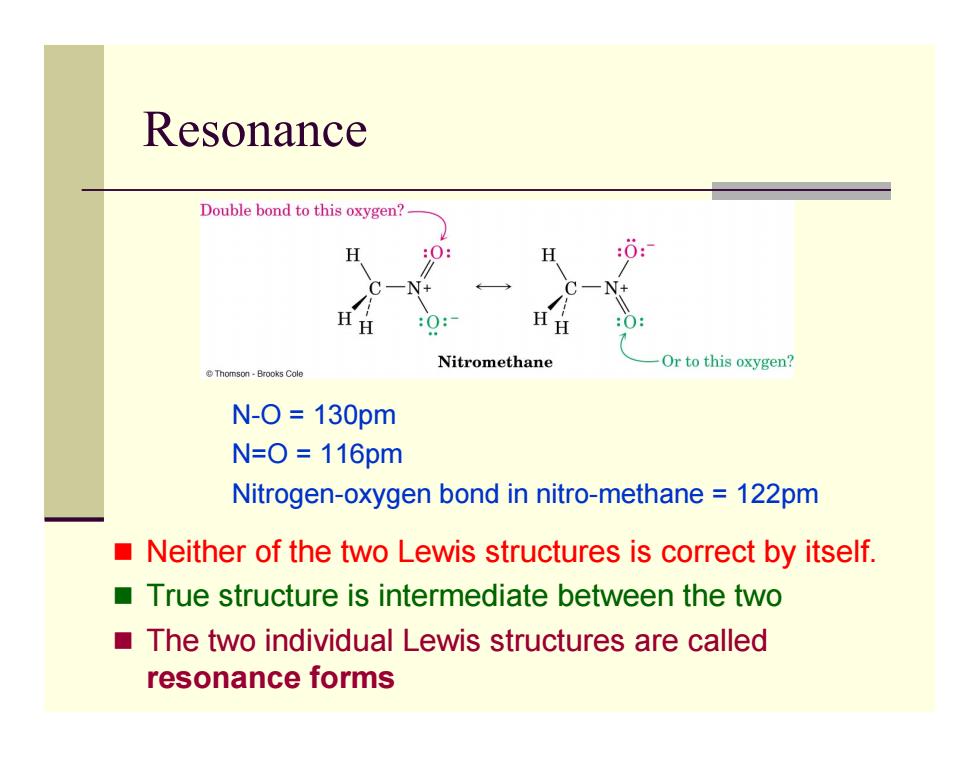
Resonance Double bond to this oxygen? H :0: Nitromethane Or to this oxygen? Thomson-Brooks Cole N-O=130pm N=0=116pm Nitrogen-oxygen bond in nitro-methane 122pm Neither of the two Lewis structures is correct by itself. True structure is intermediate between the two The two individual Lewis structures are called resonance forms
Resonance Neither of the two Lewis structures is correct by itself. True structure is intermediate between the two The two individual Lewis structures are called resonance forms N-O = 130pm N=O = 116pm Nitrogen-oxygen bond in nitro-methane = 122pm